Solve this problem by plugging the values into the Henderson-Hasselbalch equation for a weak acid and its conjugate base. Derivation of The Henderson Hasselbalch Equation.
Solved Problems Henderson Hasselbalch Equation Ph Pka Easy Biology Class
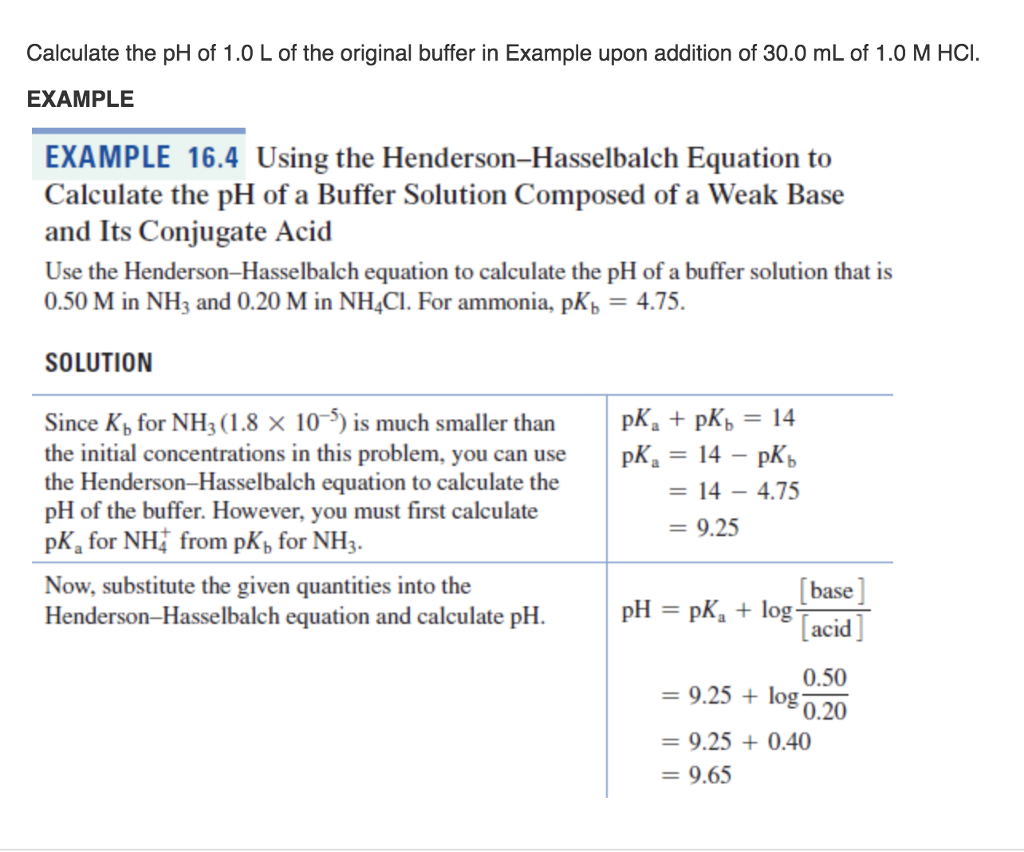
The Henderson-Hasselbalch equation is described and an example problem of how to calculate the pH of a buffer solution using the equation is given.
Henderson hasselbalch equation examples. The Henderson-Hasselbalch equation is written as. Thus we only need to convert the Ka into pKa. To derive the equation a number of simplifying assumptions have to be made.
Henderson Hasselbalch Equation Determine the pH of an aqueous solution of 10 mL of 003 M acetic acid and 15 mL of 0025 M acetate. It can be derived from the equilibrium constant expression for a dissociation reaction of the general weak acid HA in Equation 13. Henderson-Hasselbach equation A buffer is a solution that can resist changes in pH when small amounts of acid or base is added.
The formula for Henderson Hasselbalch is as follows. Contoh Soal Penerapan Persamaan Henderson-Hasselbalch. Note- Make sure the units for the A- and HA are in molar M units.
When it dissolved in water it is not completely ionised but achieves equilibrium so that it can exist both in ionized as well as unionised forms. The Henderson-Hasselbalch equation provides a relationship between the pH of acids in aqueous solutions and their pK a acid dissociation constant. In 1908 Lawrence Joseph Henderson wrote an equation that described the use of carbonic acid as a buffer solution.
PH pK a log A HA We already know the values for A- and HA. Henderson-Hasselbalch equation is a numerical expression which relates the pH pKa and Buffer Action of a buffer. Lets take a weak electrolyte such as HA which acts like weak acid.
Chemically a buffer is a solution of equimolar concentration of a weak acid such as acetic acid CH3COOH and its conjugate base such as acetate ion CH3COO. Note how decreasing the amount of base makes the buffer pH become more acidic compare to example 1. Hitung pH larutan buffer yang terbuat dari 020 M HC 2 H 3 O 2 dan 050 MC 2 H 3 O 2 -yang memiliki konstanta disosiasi asam untuk HC 2 H 3 O 2 18 x 10 -5.
PKa log Ka log 18 x 10-5 47. Later Karl Albert Hasselbalch re. Example Problem Applying the Henderson-Hasselbalch Equation Calculate the pH of a buffer solution made from 020 M HC 2 H 3 O 2 and 050 M C 2 H 3 O 2- that has an acid dissociation constant for HC 2 H 3 O 2 of 18 x 10 -5.
This chemistry video tutorial explains how to calculate the pH of a buffer solution using the henderson hasselbalch equation. For example the acid may be acetic acid and the salt may be sodium acetate. Then insert them into the Henderson-Hasselbalch Equation.
Example Problem Applying the Henderson-Hasselbalch Equation Calculate the pH of a buffer solution made from 020 M HC 2 H 3 O 2 and 050 M C 2 H 3 O 2- that has an acid dissociation constant for HC 2 H 3 O 2 of 18 x 10 -5. Here lets see how we can calculate it along with few examples. Solve this problem by plugging the values into the Henderson-Hasselbalch equation for a weak acid and its conjugate base.
Deriving the Henderson-Hasselbalch Equation. PHpK_alog_10fracA-HA Where pK_a is the acid dissociation constant A- is the molar concentration of the conjugate base and HA is the molar concentration of the weak acid. The pH of a buffer solution can be estimated with the help of this equation when the concentration of the acid and its conjugate base or the base and the corresponding conjugate acid are known.
In its general form the HendersonHasselbalch equation is a useful expression for buffer calculations. It is a mixture of a weak acid and its salt of a strong base an acidic buffer ORit is a mixture of a weak base and its salt of a strong acid a basic buffer. Now let us take a look at Henderson Hasselbalch equation derivation.
14 K H A HA where K is the equilibrium constant at a given temperature. Forms of the Henderson-Hasselbalch equation Finding the pH of a buffer made by mixing pyruvic acid with sodium pyruvate Calculating the ratio of the concentrations of acetate and acetic acid needed. Example Question 1.
Insert all knowns into Henderson-Hasselbalch equation and calculate the unknown pH. PKa of acetic acid is 475. You need to produce a buffer solution that has a pH of 5270.
A buffer is a solution which can resist the change in pH. Example Question 1. You can demonstrate that to yourself by calculating the new molarities in 0500 L them adding the two solutions together thereby cutting the molarities in half.
It explains the concept compo. Henderson Hasselbalch Equation NaOH is added to a 500mL of 2M acetic acid. You will get 4700 for your answer.
If the pK a value of acetic acid is approximately 48 what volume of 2M NaOH must be added so that the pH of the solution is 48. The HendersonHasselbalch equation relates the pH of a solution containing a mixture of the two components to the acid dissociation constant K a and the concentrations of the species in solution. PH pK a log base acid x 4752 log 0800 100 x 4752 0097 4655.
The Henderson-Hasselbalch Equation done in the Internet way. PH pK a log A HA.
Buffer Solution Ph Calculations Henderson Hasselbalch Equation Explained Chemistry Problems Youtube Teaching Chemistry Science Chemistry Buffer Solution
Chem 2 Acid Base Equilibria X Buffers And The Henderson Hasselbalc
Derivation Of Henderson Hasselbalch Equation Chemistry Stack Exchange
Ph Of Buffers Using Henderson Hasselbalch Concepts And Calculations Pt 10 Youtube
To Calculate The New Ph Use The Henderson Hasselbalch Equation Ppt Download
Henderson Hasselbalch Example Youtube
Henderson Hasselbalch Equation Youtube
Solved What I Don T Understand Is Why The Ph According Th Chegg Com
Applications And Example Problems Using Henderson Hasselbalch Equation Analytical Chemistry Pharmaxchange Info
Henderson Hasselbalch Calculator Buffer Solutions
Solved How Do I Know That 0 50 M Is Ha 0 20 M Is A Chegg Com
Henderson Hasselbalch Equation Microbe Notes
Solved Problems Henderson Hasselbalch Equation Ph Pka Easy Biology Class
Chem 2 Acid Base Equilibria X Buffers And The Henderson Hasselbalc
ads
Search This Blog
Blog Archive
- January 2023 (11)
- November 2021 (28)
- October 2021 (45)
- September 2021 (14)
- March 2021 (13)
- February 2021 (71)
- September 2020 (40)
- August 2020 (66)
- July 2020 (44)
- June 2020 (53)
- May 2020 (38)
- April 2020 (80)
- March 2020 (55)
- February 2020 (48)
- January 2020 (17)
- November 2019 (21)
- October 2019 (41)
- September 2019 (41)
- August 2019 (44)
- July 2019 (19)
- June 2019 (18)
- May 2019 (13)
- February 2019 (8)
- January 2019 (13)
- December 2018 (14)
- November 2018 (13)
- October 2018 (14)
- September 2018 (13)
- August 2018 (13)
- July 2018 (12)
Labels
- 16th
- 1800s
- 1812
- 1877
- 1966
- 1984
- 2016
- 2017
- 2022
- about
- abraham
- absolute
- abundant
- acceptance
- according
- accuweather
- acetic
- acid
- acids
- adding
- adults
- after
- alamo
- alcohol
- alexander
- alkali
- alum
- amendment
- america
- americano
- analysis
- analytic
- andrew
- animal
- animals
- answer
- answers
- anthem
- antonym
- apes
- appoint
- arachnid
- area
- argonauts
- arts
- asexual
- asilo
- assassinated
- assortment
- atom
- atomic
- attracts
- atwood
- avec
- average
- axolotl
- baby
- baking
- balance
- balanced
- balancing
- ball
- balloons
- baruch
- base
- bases
- basic
- battle
- beach
- beautiful
- become
- been
- beers
- before
- beginners
- belleau
- bend
- berkeley
- best
- between
- bibliography
- bicameral
- bicarbonate
- bien
- biggest
- bill
- billboard
- bills
- binomial
- biology
- birth
- birthday
- bites
- black
- board
- body
- boiling
- bolivar
- bond
- bonds
- book
- books
- borax
- border
- born
- boston
- bottles
- boulder
- bouncy
- brain
- brainstem
- branch
- branches
- brightest
- broken
- brook
- brown
- bugs
- bunker
- caffeine
- calculate
- caliphate
- called
- calvin
- canada
- canadian
- cannot
- capacity
- capital
- capitalism
- carbon
- card
- carol
- carolina
- caroline
- carrying
- carta
- cases
- catalyst
- catcher
- caterpillars
- caused
- causes
- celebrate
- cell
- cells
- celsius
- centigrade
- chambers
- chan
- chances
- change
- channel
- chapel
- characteristics
- characters
- charles
- chart
- cheetah
- chemical
- chemistry
- chicago
- chigger
- chiggers
- china
- chinese
- christmas
- chrome
- chromosomes
- chronological
- circumference
- cities
- citizenship
- city
- civil
- clark
- class
- classes
- classify
- clause
- clothes
- codon
- college
- colleges
- collision
- colonies
- colony
- color
- colorado
- colors
- columbia
- combined
- comes
- common
- communicate
- companies
- compare
- compared
- comparing
- completing
- complex
- components
- composition
- compound
- compounds
- comprehension
- compromise
- computer
- concentration
- conducir
- cong
- congress
- conjugation
- cons
- conscience
- consequences
- consists
- contained
- continent
- continents
- contrast
- control
- convert
- cool
- cougar
- counter
- counters
- country
- course
- covalent
- cover
- cranial
- crickets
- crime
- crossword
- crucible
- crucified
- crystals
- cubic
- curve
- cycle
- cytoplasm
- dangerous
- darwin
- date
- davis
- days
- dean
- death
- define
- definition
- degree
- density
- dependent
- depression
- describe
- dessert
- detenidas
- development
- deviation
- devil
- dictator
- difference
- different
- diffusion
- dinosaurs
- direct
- displacement
- dissolving
- distribution
- documentary
- does
- dollar
- dominican
- dominicana
- domino
- donald
- door
- double
- down
- drain
- drama
- dreams
- drinking
- duke
- dynasty
- earth
- earthworm
- ectomy
- edible
- education
- eggs
- elastic
- elected
- electoral
- electric
- electrical
- electricity
- electrolyte
- elements
- elephant
- elevation
- elie
- elizabeth
- empirical
- enable
- endoplasmic
- endothermic
- energy
- engine
- england
- english
- enterprise
- environment
- equation
- equations
- equator
- erik
- error
- escalator
- essay
- estados
- estar
- ethanol
- eukaryotes
- every
- everyday
- example
- examples
- executive
- expiration
- explain
- explanatory
- exponential
- extensive
- eyelashes
- eyes
- facts
- fahrenheit
- fair
- family
- famous
- fashion
- fast
- fathers
- fault
- federal
- feet
- fifty
- file
- find
- fire
- first
- fission
- five
- flame
- flammable
- fleas
- flies
- float
- flow
- fluoride
- flush
- food
- form
- formal
- forms
- formula
- foucault
- founded
- founding
- four
- france
- francis
- frank
- franklin
- free
- freezing
- french
- fresh
- frisbee
- from
- front
- fruit
- full
- function
- functional
- functionalist
- fused
- fusion
- gable
- games
- geek
- geese
- generations
- geography
- geologist
- german
- germany
- glue
- good
- grade
- grading
- gramophone
- grams
- great
- greek
- green
- gringo
- group
- groups
- guide
- gyri
- h2so4
- h3po4
- habit
- half
- happy
- harbor
- hard
- harm
- hasselbalch
- have
- hawaii
- heart
- heat
- height
- henderson
- hercules
- high
- highest
- hill
- hills
- hippocratic
- hiragana
- history
- hold
- holiday
- honey
- horizontal
- hornet
- host
- house
- hudson
- hula
- human
- humans
- hundred
- hurricane
- ideal
- ideas
- identify
- igneous
- immigration
- impeached
- implicit
- important
- improve
- indefinite
- independent
- index
- indicator
- indirect
- inelastic
- influence
- influenced
- influential
- instead
- institute
- institutions
- insulator
- intensive
- interaction
- intermediate
- intermolecular
- invasion
- invented
- invitacion
- ionic
- iphone
- iron
- italian
- italy
- jacket
- jackson
- japanese
- jason
- java
- jersey
- jesus
- joints
- juliet
- jupiter
- keep
- kelvin
- kicked
- kinds
- king
- kissing
- kitchen
- know
- lake
- lamp
- largest
- last
- later
- latin
- layers
- leaves
- legal
- legislative
- legislature
- legs
- length
- letter
- level
- levels
- lever
- lewis
- liberal
- libre
- licencias
- license
- life
- like
- lion
- list
- literature
- liters
- live
- llegar
- lloyd
- located
- location
- lone
- long
- love
- lowest
- lsat
- luftballons
- lynching
- lyrics
- made
- make
- makes
- making
- mandarin
- manson
- many
- margaret
- mark
- mary
- maryland
- maslow
- mass
- masses
- masters
- math
- matter
- mattress
- mcdonalds
- mean
- meaning
- medical
- mediterranean
- meet
- melt
- melting
- members
- memorandum
- memorize
- mercury
- meridian
- metals
- meters
- method
- metric
- mexico
- micron
- microsoft
- miles
- milk
- milky
- milliliters
- million
- mills
- ming
- mississippi
- missouri
- mixing
- mixtures
- mode
- model
- mojados
- mole
- molecular
- molecule
- molecules
- monologue
- mood
- moon
- morning
- most
- mountain
- movie
- much
- multiplication
- museum
- myth
- mythology
- name
- names
- narrative
- national
- natural
- navigate
- near
- nearest
- necessary
- nerves
- never
- niche
- nietzsche
- night
- nonelectrolyte
- nonpolar
- north
- nova
- novel
- nucleotide
- number
- numbers
- nurse
- obama
- occur
- official
- ohio
- oklahoma
- oldest
- olds
- only
- order
- orders
- organic
- organisms
- orleans
- osmosis
- over
- oxygen
- oxymoron
- pair
- para
- participles
- parts
- pasaporte
- past
- patches
- pearl
- penalty
- penicillin
- people
- percent
- percentiles
- perfectly
- performing
- perfume
- periodic
- peroxide
- personas
- perspective
- philip
- philosophy
- phosphorus
- photosynthesis
- physical
- pick
- pictures
- pies
- pigs
- pine
- pitzer
- plagiarism
- planets
- plans
- plant
- plastic
- plate
- play
- plow
- poem
- point
- points
- poisonous
- polar
- police
- polymer
- popular
- populated
- population
- power
- practice
- pratt
- prejudice
- prep
- president
- presidential
- presidents
- press
- pride
- prime
- principle
- printable
- probability
- products
- project
- projects
- prokaryotic
- prompts
- proof
- proper
- properties
- property
- protestant
- protons
- provinces
- pure
- purpose
- queen
- questions
- quiero
- quiet
- quiz
- quote
- quotes
- range
- ranges
- ranked
- raptors
- rate
- ratio
- reaction
- reactions
- reactive
- read
- reading
- reaper
- rebellion
- rebuttal
- recipe
- relationship
- release
- remove
- renaissance
- replace
- replacement
- replication
- report
- representational
- representative
- reproduction
- republic
- republica
- response
- reusing
- revolution
- rien
- rights
- ring
- rise
- river
- rivers
- riveter
- rocks
- rome
- romeo
- root
- rosie
- rotten
- royal
- rule
- rules
- russian
- rutgers
- salary
- salt
- saltpeter
- salvador
- sample
- sand
- santa
- scarlet
- schedule
- schools
- science
- scientific
- score
- scores
- scotia
- scratch
- seasons
- secos
- secretary
- secrets
- security
- sedimentary
- segregation
- self
- sentence
- separate
- serve
- sets
- sextillion
- shakespeare
- shark
- sharks
- shays
- shows
- silk
- simon
- simple
- single
- size
- slavery
- sleep
- slime
- slimes
- slope
- smart
- snow
- social
- socialization
- sociological
- sociology
- soda
- solution
- somatic
- song
- songs
- spanish
- speak
- species
- specific
- speech
- spell
- sphere
- spider
- spirit
- spit
- spouse
- square
- stages
- stamps
- stand
- standard
- star
- start
- state
- states
- statistics
- status
- step
- stephen
- stomata
- stony
- strands
- strategies
- strong
- strongest
- structural
- students
- study
- study material
- subject
- submarine
- substance
- summary
- superscore
- supreme
- swastika
- sweet
- symbol
- symbolic
- synthesis
- system
- systematic
- table
- tall
- taste
- taurine
- tears
- technology
- temperature
- temple
- terms
- test
- testing
- thank
- that
- themes
- theory
- these
- they
- things
- third
- thomson
- thought
- three
- tiger
- time
- today
- toddlers
- tone
- tooth
- topics
- total
- touch
- town
- trabajo
- trail
- training
- translation
- tree
- trees
- trillion
- triple
- trump
- twain
- twister
- type
- types
- ucla
- underworld
- unit
- universe
- universities
- university
- unrestricted
- upside
- using
- valentine
- value
- vaporization
- variable
- variables
- vatican
- viet
- view
- vinegar
- visa
- vive
- voila
- volcano
- vote
- voulez
- vous
- wake
- wallpaper
- warfare
- washington
- washingtons
- wasp
- wasps
- water
- wavelength
- weak
- welcome
- were
- what
- whats
- wheelbarrow
- when
- where
- which
- while
- white
- whos
- william
- wish
- with
- withdrawing
- women
- wood
- woodchuck
- woodrow
- word
- work
- worksheet
- world
- wright
- write
- writing
- written
- yang
- year
- yellow
- yield
- your
- zero
- zeros
- zippers
-
The name axolotl is also. The axolotl is an amphibious salamander Ambystoma mexicanum indigenous to Mexico. Axolotl Care Guide Housing Fee...
-
Donald John Trump was born on June 14 1946 at Jamaica Hospital in the borough of Queens in New York City. The Senate has voted to acquit the...
-
Gyri is a ridge on the surface of the brain. Sulci is a depression or groove in the cerebral cortex. Duke Neurosciences Lab 1 Surface Anat...
About Me
