It has the chemical formula CH 4. A bond composed of two electrons one from each of the two atomsThe carboncarbon single bond is a sigma bond and is formed between one hybridized orbital from each of the carbon atoms.
Why Can Carbon Form 4 Bonds Quora
If the bond is with another carbon atom it is a pure covalent or nonpolar covalent bond.
What type of bond does carbon form. The three major types of covalent bonds are single double and triple bonds. For these reasons carbon is the most essential atom for life. Carbon has a tendency to form covalent bonds.
We consider a bond to be two electrons one from each element. Carbon is able to form long stable backbones because of its small size and bond flexibility. Carbon has four valence electrons and it is only these electrons which are involved in bonding.
Nitrogen has five valence electrons and in simple amines it is trivalent with the two remaining electrons forming a lone pair. There are no atoms that pair up with each other except for Carbon and Carbon meaning there are five non-polar bond in the whole molecule. The carbon-hydrogen bond CH bond is a bond between carbon and hydrogen atoms that can be found in many organic compounds.
Two in the inner orbit and four in the outer orbit. Carbon and oxygen form terminal double bonds in functional groups collectively known as carbonyl compounds to which belong such compounds as ketones esters carboxylic acids and many more. The most common form is the single bond.
Internal CO bonds are found in positively charged oxonium ions. The polarity of a bond is determined by the difference in the electronegativities of the two atoms. An sp2 -hybridized orbital and a p-orbital that is not involved in the hybridization form a double bond while a triple bond evolves from an sp -hybridized orbital and two p-orbitals from each atom.
Most of the bonds occur between Carbon and Hydrogen leaving the difference of the Electronegativity values at3. 1 Carbon forms 4 Hydrogen forms 1 Oxygen forms 2 and N forms 3. This results in a filled outermost shell.
1 How many bonds does C H O and N form. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. 2 Briefly describe the different types of covalent bonds.
Carbon atoms form double bonds in compounds called alkenes and triple bonds in alkynes. Moreover of all the elements in the second row carbon has the maximum number of outer shell electrons four capable of forming covalent bonds. The methane molecule provides an example.
U can say. Carbon has six electrons. Redirected from Carbon-nitrogen bond A carbonnitrogen bond is a covalent bond between carbon and nitrogen and is one of the most abundant bonds in organic chemistry and biochemistry.
It requires large amount of energy to lose or gain four electronsie carbon is tetravalent so carbon prefers to share electrons rather than to lose or gain. The use of the p-orbitals forms a pi p bond. Covalent bond is a bound which is formed by the sharing of electrons in the outermost shell of carbon atom there are four electrons left which are called valence electronsvalence electrons determine the valency of an atom.
One double and two single bonds. Carbon most often forms a covalent bond with other atoms. The carbonfluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds.
This symbolizes the polarity of the bond and the bond between Carbon and Hydrogen is covalent. This bond is a covalent bond meaning that carbon shares its outer valence electrons with up to four hydrogens. This completes both of their outer shells making them stable.
A carbon atom can form the following bonds. Carbon most commonly forms covalent bonds which are two atoms sharing electrons but also sometimes forms ionic bonds found in compounds such as calcium carbide. In ethane the orbitals are sp 3-hybridized orbitals but single bonds formed between carbon atoms.
Other elements such as phosphorus P and cobalt Co are able to form five and six covalent bonds respectively with other elements but they lack carbons ability to bond indefinitely with itself. The most common oxidation state of carbon is 4 or -4. This is why carbon can form four bonds while hydrogen forms only one.
Therefore carbon atoms can form four covalent bonds with other atoms to satisfy the octet rule. If it is with another atom a polar covalent bond is formed. It is one of the strongest single bonds in organic chemistry behind the B-F single bond Si-F single bond and the H-F single bond and relatively shortdue to its partial ionic character.
Carbon is most likely to form covalent bonds because sharing four electrons with other atoms is the most energetically favorable way for carbon to fill its valence shell or obtain an octet as. A carboncarbon bond is a covalent bond between two carbon atoms.
Each carbon atom forms four chemical bonds. The hydrogen atom and the halogen atoms form only one covalent bond to other atoms in most stable neutral compounds.
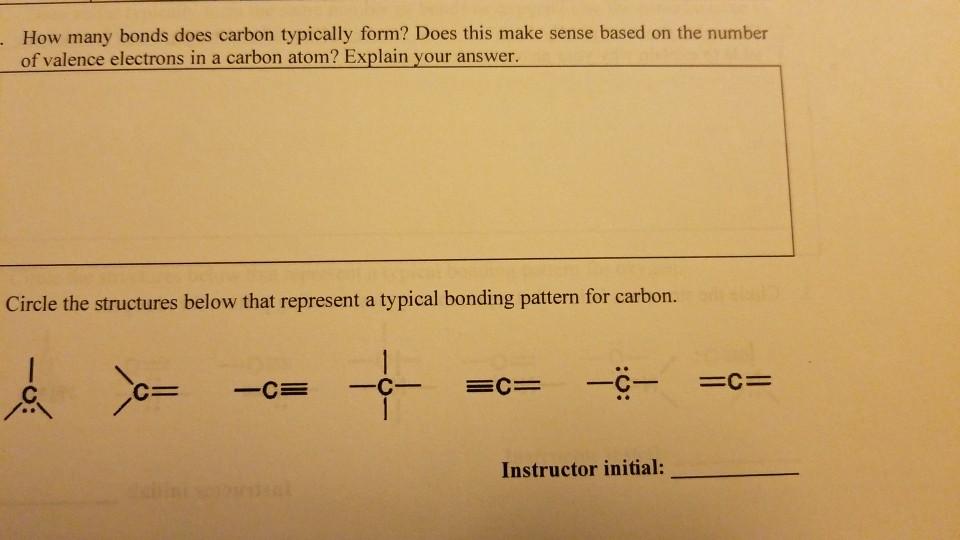 Solved How Many Bonds Does Carbon Typically Form Does Th Chegg Com
Solved How Many Bonds Does Carbon Typically Form Does Th Chegg Com
The carbon-carbon bonds in lignin are the most difficult bonds to break and many of these linkages tend to survive the pulping process 6Although carbon-carbon linkages are present in the native lignin polymer additional carbon-carbon bonds can be formed during lignin pretreatment such as in alkali-promoted condensation reactions during kraft.

How many bonds can carbon form. Please note that carbon need not always form bonds to four atoms. Therefore it can form four covalent bonds with other atoms or molecules. There are three major types of covalent bonds.
Carbon has six electrons. The methane molecule provides an example. The most common oxidation state of carbon is 4 or -4.
This coupled with the strength of the carboncarbon bond gives rise to an enormous number of molecular forms many of which are important structural elements of life so carbon compounds have their own field of study. Carbon-Carbon C C Linkages in Lignin. The simplest organic carbon molecule is methane CH 4 in which four hydrogen atoms bind to a carbon atom Figure 1.
It can do this with oxygen hydrogen or another carbon. 1 sigma bond and 1 pi bond. However in can form a bond with 5 or 6 atoms like ceFe6C fragment where iron atoms form octahedron around the carbon atom.
How many bonds can a carbon atom form. Single double and triple bonds. However the sum of orders of 6 ceC-Fe bonds will be still 4.
A carbon could form a double bond with an oxygen and then two single bonds to hydrogen atoms. This results in a filled outermost shell. Carbon most commonly forms covalent bonds which are two atoms sharing electrons but also sometimes forms ionic bonds found in compounds such as calcium carbide.
If it is with another atom a polar covalent bond is formed. Carbon is one of the few elements that can form long chains of its own atoms a property called catenation. This means that it can have up to 4 covalent bonds.
Whereas in fact it is known that the 4 C-H bonds are disposed at an angle of 10928. It has the chemical formula CH 4. Since carbon has four valence electrons it forms covalent bonds with four neighboring carbon atoms.
In these compounds carbon nitrogen oxygen and chlorine atoms have four three two and one bonds respectively. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. How many single covalent bonds can carbon form.
The most common type of bond formed by carbon is a covalent bond in which carbon shares electrons with other atoms. 1 Carbon is an element of group IVA of the periodic table and therefore it has four valence electrons. Therefore carbon atoms can form four covalent bonds with other atoms to satisfy the octet rule.
Two in the inner orbit and four in the outer orbit. Which type of bond has one pair of electrons shared between atoms. Carbon forms four bonds because it has four valence electrons.
If the bond is with another carbon atom it is a pure covalent or nonpolar covalent bond. Example CH4 - One carbon atom has combined with 4 hydrogen atoms which can only form one bond each. Carbon most often forms a covalent bond with other atoms.
Carbon cannot have more then 4 double-electron bonds in reasonable conditions. This can be accounted for if we use the concept of hybridization in functions defining the orbitals. The four electrons can be distributed throughout the four orbitals in carbons second energy level so that orbital overlap with four other elements is possible.
A single carbon atom usually can form 4 covalent bonds because it has 4 valence electrons. The reason carbon is capable of forming four strong bonds is because it has four free electrons in the outer electron shell according to Clinton Community College. Specifies which atoms are bonded to eachother.
Carbon contains four electrons in its outer shell. The situation is different if we consider exited states. A carbon atom itself can form four single bonds.
The carbon is only bonded to three atoms but is forming a total of four bonds 1 double and 2 singles. The fact that carbon atoms bond strongly with other elements prevents most carbon-based molecules from changing form at normal temperatures as explained by the University of Bristol. Other elements such as phosphorus P and cobalt Co are able to form five and six covalent bonds respectively with other elements but they lack carbons ability to bond indefinitely with itself.
Which combinations of bonds is present in this molecule. Carbon forms 4 bonds with other atoms. Now carbon atom could form 3 directed bonds which are mutually perpendicular using three 2p orbitals and one non-directed bond using 2s orbital.
The structural formula of a molecule. However structures that are more complex are made using carbon.