A measured value was 001023 C with an uncertainty of 70 µK. T C T K - 27315.
What Is The Relationship Between The Three Temperature Scales Quora
Celsius C Kelvin - 27315 If required there are worked examples below which use this formula to show how to convert a temperature in Kelvin to a temperature in Celsius.

Celsius to kelvin formula. Kelvin to Fahrenheit to Celsius or vise versa you should use the following formulas. I am unsure if it was Anders Celsius or Daniel Gabriel Fahrenheit who created this conversion formula. That is k c 27315 or c k - 27315.
T K T C 27315. This practice is permissible because the magnitude of the degree Celsius is equal to that of the kelvin. In science and in engineering the Celsius scale and the Kelvin scale are often used in combination in close contexts eg.
How to convert degrees celsius to kelvin The temperature on kelvin scale can be converted by adding 273 in the temperature Which is in degree centigrade C on Celsius scaleIts formula is given as. Kelvin to Celsius conversion formula The temperature T in degrees Celsius C is equal to the temperature T in Kelvin K minus 27315. To equal the two scales of Kelvin and Celsius you can say that the freezing point is water 273 K 0 C.
As it can be seen that 0 C is actually a much higher temperature than 0 K. Celsius To Kelvin Formula Use this formula to convert a temperature in Celsius C to Kelvin K. Use this formula to convert a temperature in Kelvin K to Celsius C.
0 C 27315 K. Kelvin K Celsius 27315 If required there are worked examples below which use this formula to show how to convert a temperature in Celsius to a temperature in Kelvin. Celsius to kelvin conversion Temperature Conversions.
To date it has not been possible to reach to absolute zero Kelvin although the approach has come close. The temperature values can be converted from one unit to another with the following formula. Also 0 Kelvin is equal to -273 degrees Celsius.
K C 27315 Your answer will be in Kelvin. The Celsius scale is based on 0 C as melting point and 100 C as the boiling point. Just enter in the fields below the value you like to convert and press the corrosponding button to the right.
Kelvin to Fahrenheit formula The degree Celsius is denoted as C and degree Fahrenheit is denoted as F The relation between Fahrenheit and Celsius is 1 C 338 F. Coexistence of Kelvin and Celsius scales. Celsius to Kelvin Conversion Formula Take your Celsius temperature and add 27315.
Type this formula in cell B2. Celsius to Kelvin Formula Celsius to Kelvin Formula The degree Celsius also known as the centigrade degree symbol as C is a thermodynamic unit defined by Anders Celsius in 1742 considering the freezing temperature of the water at 100C and the boiling temperature at 0C. Then press Enter button on the keyboard and drag the fill hand of the B2 to the end of B7 see screenshot.
Convert 20 degrees Celsius to Kelvin. T T_C 27315. 0 degrees Celsius is equal to 27315 degrees Kelvin.
Celsius to Kelvin Conversion Factor. The temperature scales used in this formula were created by Anders Celsius 17011744 and Daniel Gabriel Fahrenheit 16861736. For example 2K is known as two Kelvin.
The temperature T in Kelvin K is equal to the temperature T in degrees Celsius C plus 27315. The Kelvin temperature scale named after the first Lord Kelvin Sir William Thomson is a thermodynamic temperature scale for all universal events such that all temperatures in this scale are positive. The freezing point of water is 32 F 0 C or 273 Kelvin.
While on the other hand in Celsius scale zero is set at the freezing point of water. Similarly the Kelvin to Celsius conversion formula is T_C T - 27315. Kelvin to Celsius Celsius to Fahrenheit Fahrenheit to Kelvin Here you can convert the 3 different temperature units.
The temperature is written in terms of Celsius Fahrenheit or Kelvin. Hence to convert degree to Kelvin add 273 to Celsius degree. Celsius to Kelvin conversion formula The temperature T in Kelvin K is equal to the temperature T in degrees Celsius C plus 27315.
But a change of 1 Kelvin is equal to a change of one Celsius degree. T K T C 27315. T k 273 C 0 oC to kelvin.
Water freezes at 0 degrees Celsius which equals 273 Kelvin. If you have data is recorded as Celsius in Column A and you want the equivalent in Kelvin in Column B. Kelvin to Celsius Formula.
With the same steps you also can convert temperature units between Celsius and Fahrenheit Kelvin and Fahrenheit. The kelvin temperature scale is designed in a way that one degree Celsius and one Kelvin equal the same. Given below is the Celsius to Kelvin conversion formula.
How to convert Celsius to Kelvin.
With the help of this knowledge one can also conclude that the freezing point formula is. It is a colligative property of solutions that is generally proportional to the molality of the added solute.

The following equation is used to calculate the freezing point of a liquid.
Freezing point depression formula. LatexDelta T_f itimes K_f times molalitylatex In this equation latexDelta T_flatex is the freezing point depression Kf is the freezing point depression constant and i is the van t Hoff factor. DT f is the freezing point depression. Since the vapour pressure of a solution is less than that of pure solvent the freezing point of the solution will be lower than that of.
The freezing point depression can be calculated by the formula. It is observed that the freezing point of a solution is always less than the freezing point of the pure solvent. This kind of measurement is called cryoscopy Greek cryo cold scopos observe.
DT iK f m where DT Change in temperature in C i van t Hoff factor K f molal freezing point depression constant or cryoscopic constant in C kgmol m molality of the solute in mol solutekg solvent. Density of water at 35 C 0994 gmL K f water 186 C kgmol Solution. Formula For Depression in Freezing Point Chemistry Formulas.
We can now find the molecular weight of the unknown compound. Where DT Temperature change celsius i Vant hoff factor K f Cryoscopic constant kgmol. Freezing point is a temperature at which a solid and a liquid state of a substance have the same vapour pressure.
Thus by the knowledge of the Freezing Point Depression Coefficient one can easily obtain the freezing. DT f is the freezing point depression i is the vant Hoff factor Kf is the molal freezing point depression constant for the solvent and. Kf is the freezing point depression constant.
An equation has been developed for this behavior. Illustrating freezing point depression Students in groups of two or three can observe freezing point depression through the making of ice cream. Dt i K f m.
Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. T K f m. Observe the cold 4 and relies on exact measurement of the freezing point.
Depression in Freezing Point. Examples include salt in water alcohol in water or the mixing of two solids such as impurities in a finely powdered drug. Since saltwater will freeze at colder temperatures organisms can survive in these bodies of water.
Freezing-point depression describes the process in which adding a solute to a solvent decreases the freezing point of the solvent. This is the colligative property called freezing point depression. Molecular Weight 200 g u n k n o w n 000923 m o l 21680 g m o l.
Freezing Point total Freezing Point solvent - DT f. Both the boiling point elevation and the freezing point depression are related to the molality of the solution. The formula to calculate freezing point depression is.
The freezing point depression is the amount the freezing temperature decreases. DTf i x kf x m In this freezing point depression formula DTf is the freezing point depression i is the Vant Hoff factor kf is the cryoscopic constant and m is the molality. The formula for freezing point depression expression is.
Thus the amount of depression depends on the amount of solute added into the solution ie depends on the molarity M of the solution. The freezing point may be defined as the temperature at which the liquid and solid states of a substance have the same vapor pressure. The Freezing point depression takes place when we add a new compound into the liquid to reduce its freezing point.
Mole depression constant - formula Mole depression constant. Looking at the formula for the boiling point elevation and freezing point depression we see similarities between the two. The depression in the freezing point of a solution can be described by the following formula.
A a DT f iKfm a a. Freezing Point Depression Formula Freezing point depression can be calculated using the Clausius-Clapeyron equation and Raoults law. Freezing point depression the freezing point goes down occurs when solute is added to the pure solvent.
To find the temperature change elevation of a solvent by a solute use the freezing point depression equation. This is the freezing point lowering effect. The materials required and procedures are as follows.
The more solute dissolved the greater the effect. M is the molality of the solution. Where T is the freezing point.
K f R T 2 1 0 0 0 L. Freezing point depression is the decrease of the freezing point of a solvent on the addition of a non-volatile solute and is represented as DTfKfm or Freezing point depressionMolal freezing point constantMolality. DT f iK f m.
The freezing point depression is especially vital to aquatic life. Dt is the temperature change from the pure solvents freezing point to the freezing point of the solution. The freezing point depression constant changes depending on the solvent and the van t Hoff factor accounts for the number of particles that a dissolving solute creates in solution.
Materials per group of two or three small Ziploc bag 165 cm x 149 cm. In a dilute ideal solution the freezing point is. With the formula below freezing-point depression can be used to measure the degree of dissociation or the molar mass of the solute.
This is termed as the depression in freezing point of a solution.
Two different compounds can share the empirical formula. The chemical formula will always be some integer multiple of the empirical formula ie.
 Empirical And Molecular Formulas Of Compounds Mole And Empirical Formulas
Empirical And Molecular Formulas Of Compounds Mole And Empirical Formulas
For example the molecular formula of butane is C 4 H 8 which makes its empirical formula CH 2 while the molecular formula of ethylene is C 2 H 4 which makes its empirical formula the same as CH 2.

Empirical formula vs molecular formula. Molecular formula and empirical formula are two such symbolical methods we use to represent molecules and compounds in an easy way. Then multiply that number by the EF to get the MF. The chemical formula for a compound obtained by composition analysis is always the empirical formula.
To complete this quiz you must have a periodic table and a calculator. Empirical formulas are used to describe ionic compounds and macromolecules. A For example C 6 H 12 O 6 is the molecular.
The molecular formula is most useful when you wish to know how many atoms of the elements are present in the compound. Eg H2O empirical formula H 20 and C 6H6 empirical formula CH. To get the molecular formula you must divide the molar mass of the empirical formula into the given molecular formula mass to find the multiplier.
Empirical formula of a compound gives the simplest whole number ratio of atoms of each element present in the compound. In chemistry we often use symbols to identify elements and molecules. For some molecules the empirical and molecular formulas are the same.
The molecular formula is especially important when you start to work with organic chemistry. The key difference between empirical and molecular formulas is that an empirical formula only gives the simplest ratio of atoms whereas a molecular formula gives the exact number of each atom in a molecule. The molecular formula is commonly used and is a multiple of the empirical formula.
The molecular weight is given. Molecular vs Empirical Formula. Molecular formula is the actual representation of the elemental composition of the compound.
The molecular formula shows the actual number of atoms of each element present in a. Calculate the empirical formula of NutraSweet and find the molecular formula. This quiz covers simple empirical and molecular formula calculations.
Integer multiples of the subscripts of the empirical formula. Molecular formula of a compound gives the actual number of atoms of each element present in one molecule of the compound. It gives more information than the empirical formula and is therefore more common.
The molar mass of NutraSweet is 29430 gmol Start with the number of grams of each element given in the problem. The molecular formula gives the actual whole number ratio between elements in a compound. A molecular formula demonstrates the number of each atom present in a given molecule.
Therefore the molecular formula is twice the empirical formula. Molecular formulas are able to describe a wide range of molecules as they give no information about the structure. The empirical formula is CH3O so the empirical formula weight is 1201 3 1008 1600 3103.
Two different compounds can share the empirical formula. NutraSweet is 5714 C 616 H 952 N and 2718 O. If you can divide all of the numbers in a molecular formula by some value to simplify them further then the empirical or simple formula will be different from the molecular formula.
Difference Between Empirical and Molecular Formula Definition. The empirical formula of a compound is the simplest whole number ratio of atoms of each element in a compound. The empirical formula for glucose is CH 2 O.
Glucose has 2 moles of hydrogen for every mole of carbon and oxygen. We can obtain the chemical formula from the empirical formula if we know the molecular weight of the compound. The molecular formula of any compound is always fixed except isomerism.
The empirical formula of a compound gives the simplest ratio of the number of different atoms present whereas the molecular formula gives the actual number of each different atom present in a molecule. A compound formed through the bonding of 2 or more atoms. The molecular formula lists all the atoms in a molecule while the empirical formula shows the ratio the number of the atoms in a molecule.
We will talk about what empirical formula and molecular formula are how they are different and well learn how to write the empirical formula for a compoun. However these are two different compounds. If the formula is simplified then it is an empirical formula.
The molecular formula of any compound sometimes becomes identical with empirical formula and sometimes it becomes multiple of the empirical formula. However these are two different compounds. Empirical formula is the simplest form of expressing the elemental composition of a compound.
The general flow for this approach is shown in Figure PageIndex1 and demonstrated in Example PageIndex2. C 2 H 6 O 2. The formulas for water and hydrogen peroxide are.
An integer that is multiplied by the empirical formula in order to obtain the molecular formula. Empirical and Molecular Formula Key Takeaways The empirical formula gives the smallest whole number ratio between elements in a compound. For example the molecular formula of butane is C 4 H 8 which makes its empirical formula CH 2 while the molecular formula of ethylene is C 2 H 4 which makes its empirical formula the same as CH 2.
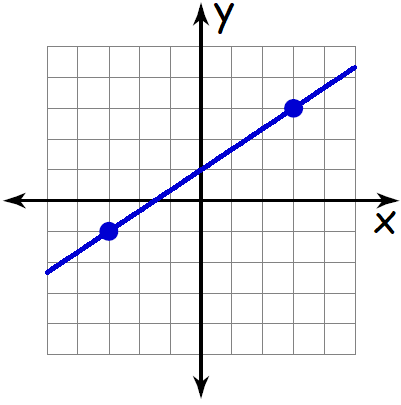
Slope rise run. The Pitch value can be obtained by dividing N by 12 and divide S by the answer.
Slope - 32.
Rise over run formula. It is described by the gradient formula. The diagram shown below illustrates this. With rise y₂ - y₁ and run x₂ - x₁.
If you dont want to use one of these preprogrammed values the calculator allows you to determine the ramp length and slope for any given rise and run ratio. This page offers you the rise and run formula to find the pitch slope angle of the roof of a building. Your goal is to find the change in the height of the line over the horizontal distance of the line.
Today were going to look at slope the grade of a straight line on the coordinate plane. Slope - 64. The simplest way to look at the slope is.
RampLength2 RampRise2 RampRun2. Updated September 26 2019. Slope is essentially change in height over change in horizontal distance and is often referred to as rise over run It has applications in gradients in geography as well as civil engineering such as the building of roads.
The rise of a road between two points is the difference between the altitude of the road at those two points say y 1 and y 2 or in other words the rise is y 2 y 1 Dy. Angle Percentage 6 10 x 100. A description of what pitch is and how to manipulate the numbers when framing and planning a project.
Slope measures the angle of change in elevation which can be obtained by dividing rise by run and multiply the result with 100. Pitch S N 12 Slope S N 100 Angle tan-1 S N Where S Rise inches N Run inches. In the case of a road the rise is the change in altitude while the run is the difference in distance between two.
2 If we know the angle of a road or highway for example 3 degrees then the ratio 1 in 1 tan A which equals 1 in 1 tan 3. All you need to do is calculate the difference between the two points in the vertical direction rise and then divide it by the difference in the horizontal direction run. Asi como calculas la elevacion de una linea en tu clase de geometria para calcular la inclinacion de una ruta solo tienes que dividir la pendiente por el trayecto.
Carpentry is mostly breaking down the elements of a bui. To find the percentage of a slope use this slope percent formula. Specifically were looking at how to use the rise over run formula to measure a lines slope.
Similar to calculating the slope of a line in your geometry class calculating the incline of a road is simply rise over run. Angle A arctangent rise run which equals arctangent 1 20 arctangent 05 28624 degrees and the grade rise run 100 which equals 1 20 100 5. For example in the equation the slope would be.
The rise is how much higherlower the second point is from the first and the run is how far horizontally they are from each other. Slope Percent Amount of Rise Amount of Run x 100. How do you find the percent of slope using rise over run.
Gradient rise run. M stands for slope. You can still think of this slope in terms of rise over run if you turn it into a fraction.
The slope formula is sometimes called rise over run The simple way to think of the formula is. RISE OVER RUN FORMULA. The rise-over-run formula is quite literally that rise over run as that is how you would calculate the slope of a straight line joining any two points.
We want to see how they relate to each other that is what is the rise over run ratio between them. You can rearrange the equation of a line into this formula to find the slope. The angle percent of a slope with rise of 6 units and run of 10 units is.
The formula for slope is referred to rise over run Because the fraction consists of the rise the change in y going up or down divided by the run the change in x going from left to the right. Find the slope of the line shown below using rise over run formula. For relatively short distances where the earths curvature may be neglected the run is the difference in distance from a fixed point measured along a level.
In this formula equals the slope of the line. RampLength sqrt RampRise2 RampRun2. The formula for slope is sometimes referred to as rise over run because the fraction onsists of the rise being the change in y going up or down divided by the run being the change in x going from left to the right.
This is sometimes also called the slope formula which states that slope change in y change in x or y1 y2 x1 x2.
On the otherhandthe vinegar is 6-10 Acetic acidThe formula for vinegar will be CH₃COOHThe IUPAC name for acetic acid is ethanoic acid. When baking soda and vinegar combine they react creating water and carbon dioxide.
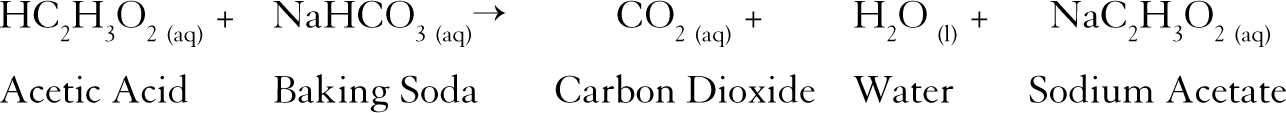 A Chemistry Puzzle To Be Solved Nsta
A Chemistry Puzzle To Be Solved Nsta
Little is known about the safety of taking both together so it may be safest to avoid.

Vinegar and baking soda formula. NaHCO 3 HC 2 H 3 O 2 NaC 2 H 3 O 2 H 2 O CO 2. Baking soda is a base also known as Sodium Bicarbonate and has the chemical formula NaHCO3. Measure out 1 cup 240 mL of rubbing alcohol 1 cup 240 mL of water and 1 tablespoon of white vinegar.
Baking soda and vinegar are best cleaners for your toilet. The baking soda and vinegar volcano is a fun chemistry project you can do to simulate a real volcanic eruption or as an example of an acid-base reactionThe chemical reaction between baking soda sodium bicarbonate and vinegar acetic acid produces carbon dioxide gas which forms bubbles in dishwashing detergent. Great addition to diaper pails.
The chemical equation for the reaction is Sodium bicarbonate and vinegar Sodium acetate and water and carbon dioxide NaHCO 3 CH 3 COOH CH 3 COO - Na H 2 O CO 2. Mix baking soda bicarbonate of soda and water to form a paste. The science behind baking soda bottle rockets.
Baking soda is essentially a base that reacts with other things that have acid content such as yogurt vinegar and buttermilk and releases carbon dioxide in the form of bubbles. Carbon dioxide bubbles through the liquid in the drain just as it does in a carbonated beverage. The reaction between baking soda and vinegar actually occurs in two steps but the overall process can be summarized by the following word equation.
Spread this paste all over the inside of your oven remove the racks avoiding the heating elements. Similar to yesterdays experiment we are working with an acid base chemical reaction in this project. In our case baking soda is sodium bicarbonate a base and vinegar is diluted acetic acid.
Brunette says to sprinkle 4 tablespoons of baking soda followed by two cups of vinegar. The ideal proportion is the following. Sprinkle baking soda on carpets let stand for 15-20 minutes and vacuum to remove odors.
A pinch of baking soda. For it to be truly effective it should be raw or unpasteurizedAs a result you will benefit more from all of its properties. Pour ½ cup of vinegar over sprinkled baking soda.
The baking soda and vinegar solution works in similar fashion to some commercial foaming drain cleaners but without the harsh chemicals. Try to get organic and high quality baking soda. Baking soda vigorously reacts with vinegar to produce carbon dioxide gas.
7Baking Soda and Vinegar Cleaning Solution for Toilet Bowl Cleaner. Vinegar or Acetic Acid has the chemical formula CH3COOH. Although both of these are leavening agents these release the trapped carbon dioxide in the batter or dough which subsequently makes it rise but baking soda.
Baking soda means sodium bicarbonate or sodium hydrogen carbonate. Baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion. For a DIY bathroom cleaner combine 1 23 cup of baking soda with 12 cup of dish soap in a bowl.
This activity explores the popular baking soda and vinegar reaction which is a simple acid-base chemical reaction. Baking soda is a base also known as Sodium Bicarbonate and has the chemical formula NaHCO3. Baking soda and apple cider vinegar may interact with medications and cause side effects of varying severity.
Leave an open box of baking soda in. Sprinkle baking soda on the dog and brush. Sprinkle ½ cup of baking soda in the toilet.
A glass of warm or hot water because cold water could be harmful to your liver. The balanced chemical equation is. The chemical formula for baking soda is NaHCO₃.
When they react to release the OH and H to become water they also release carbon dioxide. Sodium acetate is made of 1 sodium ion 2 carbon atoms 3 hydrogen atoms and 2 oxygen atoms. When the vinegar and baking soda react one of the bi-products of the reaction is the production of carbon dioxide gas.
This is how the baking soda and vinegar tip is supposed to work. Stir in 12 cup of water followed by 3 tablespoons of vinegar and continue mixing to combine the ingredients and get rid of any lumps. Once the bubbling stops flush the drain with boiling water.
Soak diapers in a solution of baking soda before washing to remove odors. Balanced Chemical Equation for Baking Soda and Vinegar Reaction One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate one mole of water and one mole of carbon dioxide. Spray the mixture onto glass mirrors ceramic tiles and chrome finishes then wipe with a paper towel or microfiber cloth.
Vinegar or Acetic Acid has the chemical formula CH3COOH. Here is a toilet bowl cleaning procedure. Let the bubbles subside for 10 minutes.
A tablespoon of organic apple cider vinegar. Scrub it with a toilet brush. This makes the reaction bubble and expand just like when you shake up a can of soda and open it.
Sprinkle baking soda in trashcans before and after adding trash bags. Pour them into a spray bottle. During this reaction the products are sodium acetate C2H3NaO2.
Heres how to clean your drain. During this reaction the products are sodium acetate C2H3NaO2. To easily apply this cleaner to any bathroom surface put it in a squirt bottle.