Covalent bonds form between non-metal atoms. Difference between ionic and covalent compounds properties The properties of covalent compounds differ considerably from those of ionic compounds.
 Covalent Compounds Covalent Bond Properties Examples With Videos
Covalent Compounds Covalent Bond Properties Examples With Videos
Most of these liquids are organic compounds.

Properties of covalent compounds. STATE Due to weak intermolecular forces generally covalent molecules or covalent compounds are liquids and gases. Explained Properties of Covalent Molecular Compounds Introduction to Covalent Bonds The formation of ionic bond is possible only when there is a large difference in the electronegativities of the combining atoms. Description of the properties of covalent compounds for grade 10 science.
However some like carbon compounds can be very large. Most covalent compounds have relatively low melting points and boiling points Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds. Due to the sharing of electrons they exhibit characteristic physical properties that include lower melting points and electrical conductivity compared to ionic compounds.
Properties of covalent compounds 1Covalent compounds exist as single molecules which are mostly in gaseous state hydrogenoxygen ammoniaSome of them may be liquid bromine and few as solids iodine phosphorus 2Covalent compounds have usually low melting and boiling pointSince no ions are present in the covalent molecules. A molecule is a group of two or more atoms joined together by covalent bonds. Uses of covalent compounds as solvents.
Covalent compounds tend to be more flammable than ionic compounds. However some covalent substances are solids. Covalent compounds have bonds where electrons are shared between atoms.
Covalent compounds in the form of liquids are mostly used as solvents in our daily life. They are known as organic solvents. Covalent bonds only form between nonmetallic elements because these elements have the same or similar electronegativity values.
Flammability is a general property of covalent compounds because a large majority of the known covalent compounds are organic. Many covalent compounds have low melting and boiling points. STATE Due to weak intermolecular forces generally covalent molecules or covalent compounds are liquids and gases.
PROPERTIES OF COVALENT COMPOUNDS MOLECULAR FORM Covalent compound exists as a separate molecules because they are formed by neutral atoms they are electrically neutral and the forces of attraction between these molecules is small. Covalent compounds have low melting and boiling points. Since most organic compounds burn we can safely list this as a property of covalent compounds even though there are many covalent compounds that dont burn.
An example is the diamond in which carbon atoms each share four electrons to form giant lattices. The properties of covalent compounds. Properties of Covalent Compounds Most covalent compounds have relatively low melting points and boiling points.
The properties of a covalent bond are determined by the exact shape of the orbitals and how those waves combine with each other. Most covalently bonded substances consist of small molecules. Covalent compound exists as a separate molecules because they are formed by neutral atoms they are electrically neutral and the forces of attraction between these molecules is small.
Here is a short list of the main properties of covalent compounds. Examples of Covalent Compounds. No ionic bonding is possible when atoms of similar electronegativities combine together.
Covalent compounds generally have low boiling and melting points and are found in all three physical. Molecules of the same element or compound always contain. The orbitals will combine and take on a shape that minimizes the potential energy between the atoms.
Hence they exist as liquids at room temperature and are volatile. While the ions in an ionic compound are strongly attracted to each other covalent bonds create molecules that can separate from each other when a lower amount of energy is added to them. Chemical bonding and its types.
Here are examples of covalent compounds and a look at their common properties. Each bond consists of a shared pair of electrons and is very strong. As I just mentioned a second ago the properties of covalent compounds are dominated by the fact that molecules arent really attracted to one another.
Covalent compounds or molecular compounds are chemical compounds made of elements connected by covalent bonds. Covalent bonds between atoms are quite strong but attractions between moleculescompounds or intermolecular forces can be relatively weak. A comparison of the properties of ionic and covalent compounds is given below.
Several physical properties of moleculescompounds are related to the presence of covalent bonds. Simple molecular substances and giant covalent structures have different. Since most covalent compounds contain only a few atoms and the forces between molecules are weak most covalent compounds have low melting and boiling points.
About Covalent and Ionic Bonds. Covalent bonds are molecules that are mostly gases or liquids.
 Difference Between Ionic Bond And Covalent Bond Youtube
Difference Between Ionic Bond And Covalent Bond Youtube
Covalent bond compounds are insoluble in water and other polar solvents.
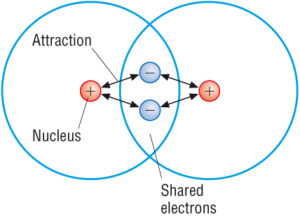
What is the difference between ionic and covalent bonds. In covalent bonds atoms are electrostatically attracted within the course of each other whereas in ionic bonds. The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the sharing is so unequal that an electron from atom A is completely lost to atom B resulting in a pair of ions. Ionic bonds form between a metal and a nonmetal.
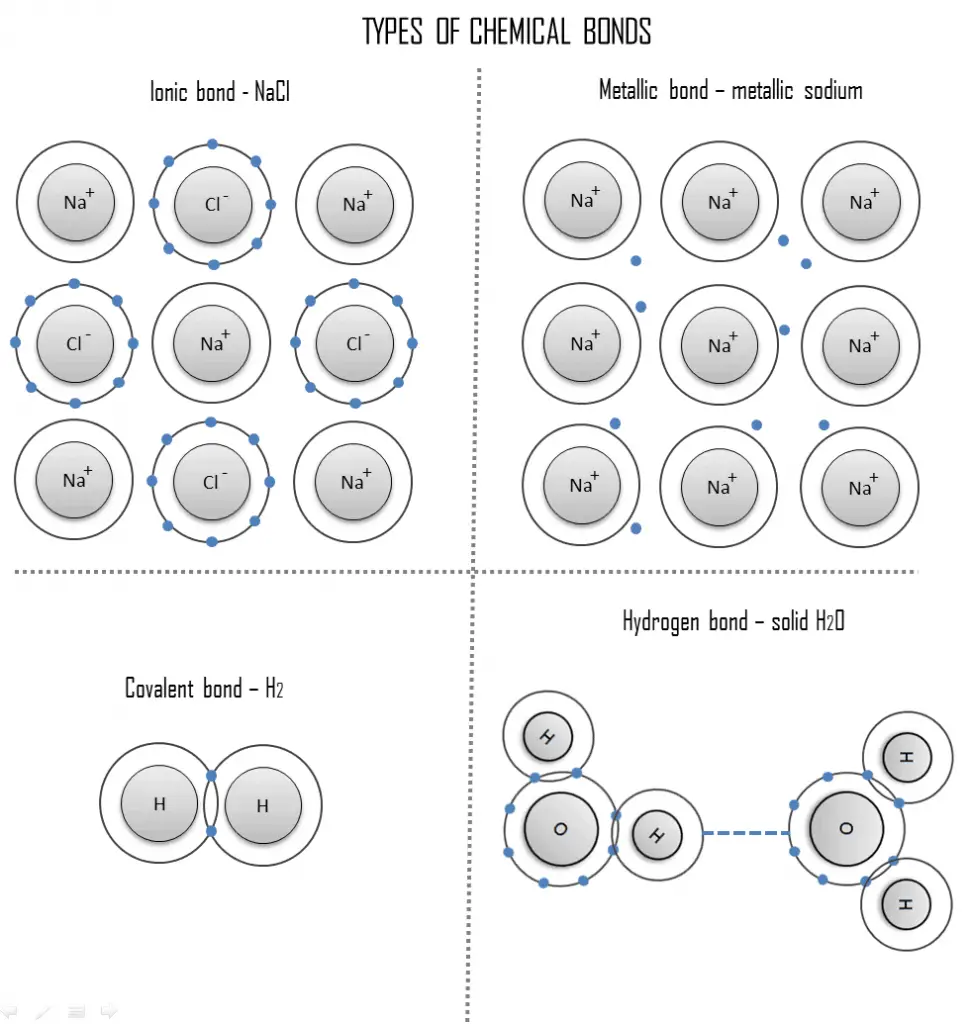
The rate of reaction of covalent bonds is comparatively low whereas that of an ionic bond is comparatively instantaneous. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. Covalent bond involves the sharing of electrons while metallic bonds have strong attractions and ionic bonds involve the transferring and accepting of electrons from the valence shell.
The definition of the chemical bond as a shared electron pair could be extended to describe the dative bond and the elaboration of Lewis acidbase interactions. Ionic bonds exist between metals and non-metals. An ionic bond essentially donates an electron to the other atom participating in the bond while electrons in a covalent bond are shared equally between the atoms.
Ionic bond compounds are soluble in water and other polar solvents. After they link together they are connected. Advertisement - Continue Reading Below Contents.
Ionic and covalent bonds are fundamentally different in the way they are formed. In an ionic bond it involves atoms linking together. In covalent bonds atoms are electrostatically attracted towards each other while in ionic bonds.
Each atom consists of protons neutrons and electrons. This is the underpinning difference between ionic and covalent bonds because normal covalent natured relationships feature harmonious electron attachments but here they synchronise with one atom only. Difference Between Ionic Covalent and Metallic bonds The attractive force which holds together the atoms or group of atoms in a chemical species is known as a chemical bond.
Difference between Covalent Bonds and Ionic Bonds. Ionic bonds dissociate in water to result in ions whereas covalent bonds do not dissociate in water. Ionic bonds do.
Due to the difference in bonding styles covalent bonds can form between atoms of the same element such as hydrogen gas which has the formula H 2 but ionic bonds cant. Ionic is a type of chemical bond where atoms are bonded together by the attraction between opposite charges. The molecules that make up living things are covalently bonded for example and covalent bonds are more common in nature than ionic bonds overall.
Covalent bonds form between two similar non-metals. Covalent bonds and ionic bonds are two different ways of how elements bond to each other. Electron pairs are shared between atoms.
The main difference between ionic bonds and covalent bonds is sharing of electron pairs and atoms. Electron pairs are shared between atoms. But there is so much more to learn about ionic vs covalent read on to find out more.
Covalent bonds are poor conductors whereas ionic bonds are good conductors in a molten state. Covalent and ionic are two types of chemical bonds. Differences between ionic and covalent bonds Covalent bonds are much more common in organic chemistry than ionic bonds.
The main difference between ionic bonds and covalent bonds is sharing of electron pairs and atoms. In this bond the electrons are moved from one bond to another. The end result is two atoms featuring separate positive and negative charges.
The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. The only pure covalent bonds occur between identical atoms. Ionic bond compound are only poor conductors in solid state but they are good conductors in molten state or in solution form.
However by doing this both bonds create elements that are neutrally charged making them stable compounds. Covalent is a type of chemical bond where atoms are bonded together by the sharing of electrons. HowStuffWoks explains ionic and covalent bonds.
This is done through a force that is electrostatic. Covalent bonds form between two nonmetals. Find more free tutorials videos and readin.
Usually there is some polarity polar covalent bond in which the electrons are shared but spend more time with one atom than the other. Ionic bond also known as electrovalent bond is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Additionally both bonds focus on the electrons.
In covalent bonds atoms share electrons whereas in ionic bonds atoms. Covalent bond compounds are poor conductors. Covalent bond occurs between the two non-metals metallic bond occurs between two metals and the ionic bond occurs between the metal and the non-metal.
This two minute animation describes the Octet Rule and explains the difference between ionic and covalent bonds. One shares and the other trades.
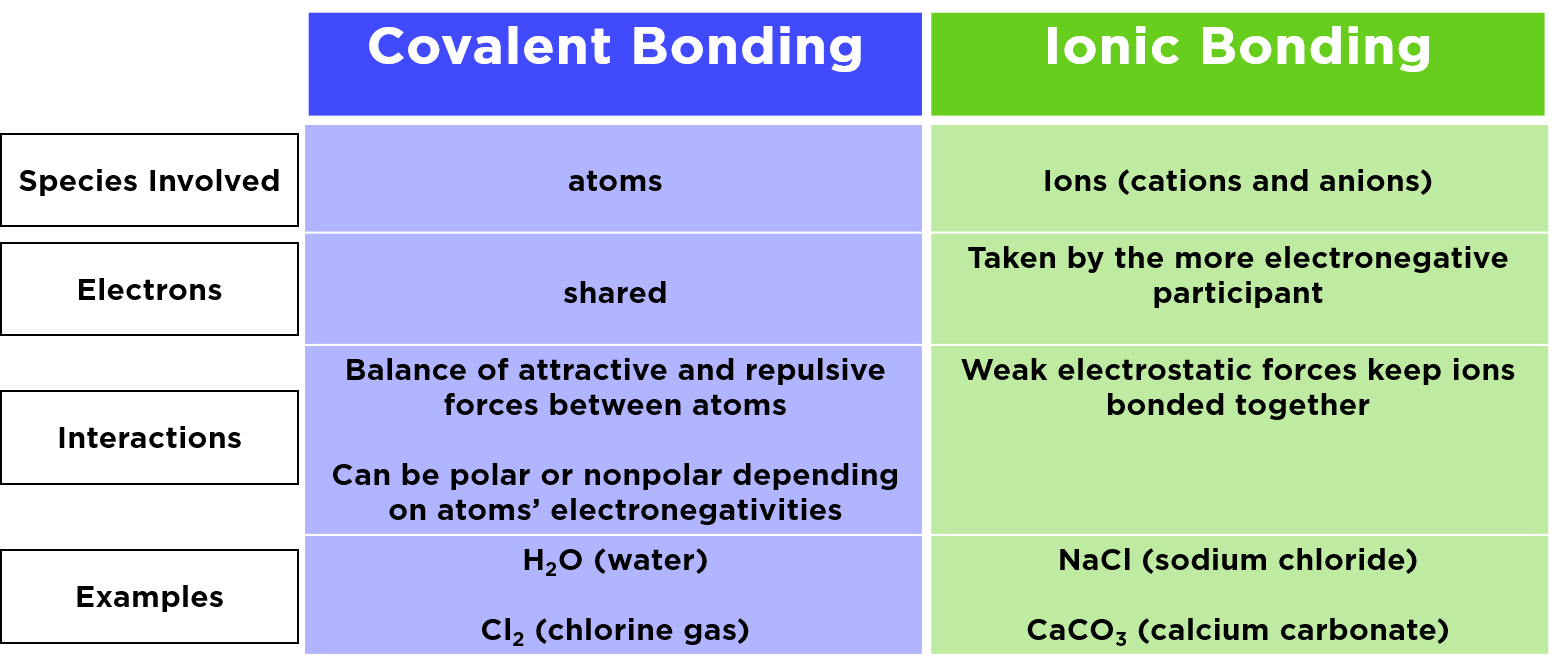
Ionic Compoundbetween a Metal and Non-MetalM NM MolecularCovalent Compoundbetween a Non-Metal and Non-MetalNM NM Metallic Bondbetween a Metal and MetalM M Determine if the elements in the following compounds are metals or non-metals. An ionic bond is formed between a metal and a non-metal.
 15 Major Difference Between Covalent And Ionic Bonds With Table Core Differences
15 Major Difference Between Covalent And Ionic Bonds With Table Core Differences
Then determine the type of bond or compound that exists between these.
Ionic vs covalent bonds. Therefore an ionic bond typically happens between metal and nonmetal. Neither atom is strong enough to attract electrons from the other. Ionic vs Covalent - Distance Background.
Covalent bonds can be found in three more subgroups as ionic bonds hydrogen bonds and Van der Waals interactions. The key difference between covalent and noncovalent bonds is that covalent bonds form when two atoms share their electrons with each other whereas noncovalent bonds form either by completely exchanging electrons between two atoms or by not exchanging any electron. Ionic Compounds have very high boiling as well as melting points while the Covalent Compounds have low melting and boiling points.
An ionic bond essentially donates an electron to the other atom participating in the bond while electrons in a covalent bond are shared equally between the atoms. Covalent bond involves the sharing of electrons while metallic bonds have strong attractions and ionic bonds involve the transferring and accepting of electrons from the valence shell. In covalent bonds atoms are electrostatically attracted within the course of each other whereas in ionic bonds.
If each of the two atoms shares an electron with the other atom nearly equally the bond is called covalent. Ionic bonds form solids at room temperature whereas covalent bonds form liquids or gasses. Covalent bonds are formed between two non-metals whereas ionic bonds are formed between a metal and non-metal.
Ionic and covalent bonds comprise the only two types of atomic bonds. This two minute animation describes the Octet Rule and explains the difference between ionic and covalent bonds. Electron pairs are shared between atoms.
This works best when the atoms in question have similar electronegativity values which is to say the strength with which they each attract other atoms and hold shared electrons is pretty equal. The two main types of chemical bonds are ionic and covalent bonds. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them.
Lets go through each. A chemical bond is the glue or force of attraction that holds two ions or atoms together. The only pure covalent bonds occur between identical atoms.
Each atom consists of protons neutrons and electrons. Bonds are generally classified into two types. The Ionic Compounds exist in the solid-state while Covalent Compounds exist in Solid Liquid and Gaseous state.
Find more free tutorials videos and readin. The ionic bond is the bond in which an electron leaves one atom to join another while the covalent bond is the bond in which there is a sharing of an electron with two atoms. It takes place been similar atoms ie.
A covalent bond is formed between two non-metals that have similar electronegativities. What dictates which kind of bond will form. An ionic bond is a chemical bond between two dissimilar ie.
Electronegativity values of course. About Covalent and Ionic Bonds The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the sharing is so unequal that an electron from atom A is completely lost to atom B resulting in a pair of ions. For stabilization they share their electrons from outer molecular orbit with others.
Ionic bonds form solids at room temperature whereas covalent bonds form liquids or gasses. Covalent bonds have a definite shape while ionic bonds do not have a definite shape. Ionic Bond Covalent Bond James Bond so many bonds.
Covalent bond occurs when atoms share their outer shell electrons with each other while ionic bond occurs when one atom donates an electron to another atom Covalent bond have low polarity while ionic bond has a high polarity Ionic bond has no definite shape while covalent bond has a definite shape. This is not always the case however. Ionic Compounds mostly are soluble in water while the Covalent Compounds are not.
Chemical bonds form between two atoms each with its own electron environment. Ionic bonding is an attraction between oppositely charged ions resulting from an exchange of electrons. Covalent bond occurs between the two non-metals metallic bond occurs between two metals and the ionic bond occurs between the metal and the non-metal.
Molecules formed by covalent bonds have a low melting point whereas those with ionic. Two of the strongest forms of chemical bond are the ionic and the covalent bonds. A covalent bond is another strong chemical bond.
The main difference between ionic bonds and covalent bonds is sharing of electron pairs and atoms. A metal and a non-metal atoms in which one atom gives up an electron to another. The melting point of covalent bond is low and ionic bond is high.
Difference between Ionic and Covalent Bond Key difference.
Nonpolar covalent bonds are very powerful bonds demanding a large amount of energy to break the bond. Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they also uniformly share the electrons.
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally.

Nonpolar covalent bond examples. An example is H 2. B The fluorine atom attracts the. Figure PageIndex1 Polar versus Nonpolar Covalent Bonds.
A bond in which the electronegativity difference is less than 19 is considered to be mostly covalent in character. Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they also equally share the electrons. For example molecular oxygen O 2 is nonpolar because the electrons will be equally distributed between the two oxygen atoms.
Technically nonpolar bonding only occurs when the atoms are identical to each other eg H 2 gas but chemists consider any bond between atoms with a difference in electronegativity less than 04 to be a nonpolar covalent bond. This is a nonpolar covalent bond. Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they also equally share the electrons.
These compounds are called non-polar covalent compounds. An example of a nonpolar covalent bond is the bond between two hydrogen atoms because they uniformly share the electrons. Hydrogen Molecule H2 is a non-polar covalent bond example as an electron pair is equally shared between the two hydrogen atoms.
A Polar Covalent Bond is unequal sharing of electrons between two atoms Polar bonds are form when there is a different between the electronegativity values of the atoms participating in a bond Electron density is distributed asymmetrically throughout the molecule EXAMPLES. An example of a nonpolar covalent bond is the bond between two hydrogen atoms because they equally share the electrons. In a nonpolar covalent bond the electrons are evenly distributed.
Nonpolar covalent bonds are remarkably important in biology. The four bonds of methane are also considered to be nonpolar. Examples For Non-Polar Solids.
These can exist as solids and liquids. What distinguishes nonpolar covalent bonds is that their electrons are shared equally. For example molecular oxygen O 2 is nonpolar because the electrons will be equally distributed between the two oxygen atoms.
The common examples are He Ne Ar Benzene H 2 N 2 O 2 Cl 2 Carbon dioxide Methane etc. Polarization of Covalent Bonds. Non-polar covalent bonds with equal sharing of the bond electrons arise when the electro-negativities of the two atoms are equal.
However at this point we need to distinguish between two general types of covalent bonds. Technically nonpolar bonding only occurs when the particles are identical to each other eg H2 gas. Due to this there is a permanent.
A nonpolar covalent bond is a covalent bond in which the bonding electrons are shared equally between the two. Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally. The two most notable types of covalent bonds are polar covalent bonds and purenonpolar covalent bonds.
Nonpolar covalent bonds are very strong bonds requiring a large amount of energy to break the bond. Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they also equally share the electrons. What is Polar molecule.
The four bonds of methane are also considered to be nonpolar. A covalent bond that has an equal sharing of electrons part a of Figure PageIndex1 is called a nonpolar covalent bond. Another example of a nonpolar covalent bond is the bond between two chlorine atoms because they also equally share the electrons.
A The electrons in the covalent bond are equally shared by both hydrogen atoms. Gas where H H both have same electro-negativities. It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond.
In general if the electronegativity difference between two atoms is less than 05 the bond is considered nonpolar even though the only truly nonpolar molecules are those formed with identical atoms. Differences Between Polar And Non-Polar Covalent Solids. You can predict nonpolar molecules will form when atoms have the same or similar electronegativity.
Pure covalent bonds nonpolar covalent bonds share electron sets equally between atoms. All these do not show polarity in their bonds instead they show zero dipole moment. For example molecular oxygen O 2 is nonpolar because the electrons will be equally distributed between the two oxygen atoms.
In a Nonpolar covalent bond the distribution of electrical charge is balanced between the two atoms. Another example of a nonpolar covalent bond is methane CH 4 also shown in Figure 1Carbon has four electrons in its outermost shell and. An example of a nonpolar covalent bond is the bond between two hydrogen atoms because they equally share the electrons.
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally. Pure covalent bonds nonpolar covalent bonds share electron pairs equally between atoms. Still chemists consider any bond between atoms with a difference in electronegativity less than 04 to be a nonpolar covalent bond.
Examples of covalent compounds include DNA water and sucrose. HH Nonpolar Covalent Bonds. Example Nonpolar Covalent Bond is found in gas molecules like Hydrogen gas Nitrogen gas etc.
