For example tossing table salt sodium chloride onto ice when its 0F wont do anything more than coat the ice with a layer of salt. The freezing point of the water is lowered once the salt is added so it the salt makes it more difficult for water to freeze.
 How Does Salt Melt Ice And Make Ice Cream
How Does Salt Melt Ice And Make Ice Cream
When the salt mixes with this thin water layer it decreases its freezing point.

Adding salt to ice. When you put salt on ice cubes they start to melt but at the same time they feel colder. Students add salt to ice to see what happens to the melting point. In both cases the salt works by lowering the melting or freezing point of water.
Salt Lowers the Temperature of Ice Water. For example tossing table salt sodium chloride onto ice when its 0F wont do anything more than coat the ice with a layer of salt. When the ionic compound salt is added to the equation it lowers the freezing point of the water which means the ice on the ground cant freeze that layer of water at 32 F anymore.
You can either add rock salt to a cooler full of ice or you can actually make ice by freezing salt water. Salt is also used to make homemade ice cream. The salt dissolves and the water becomes salty.
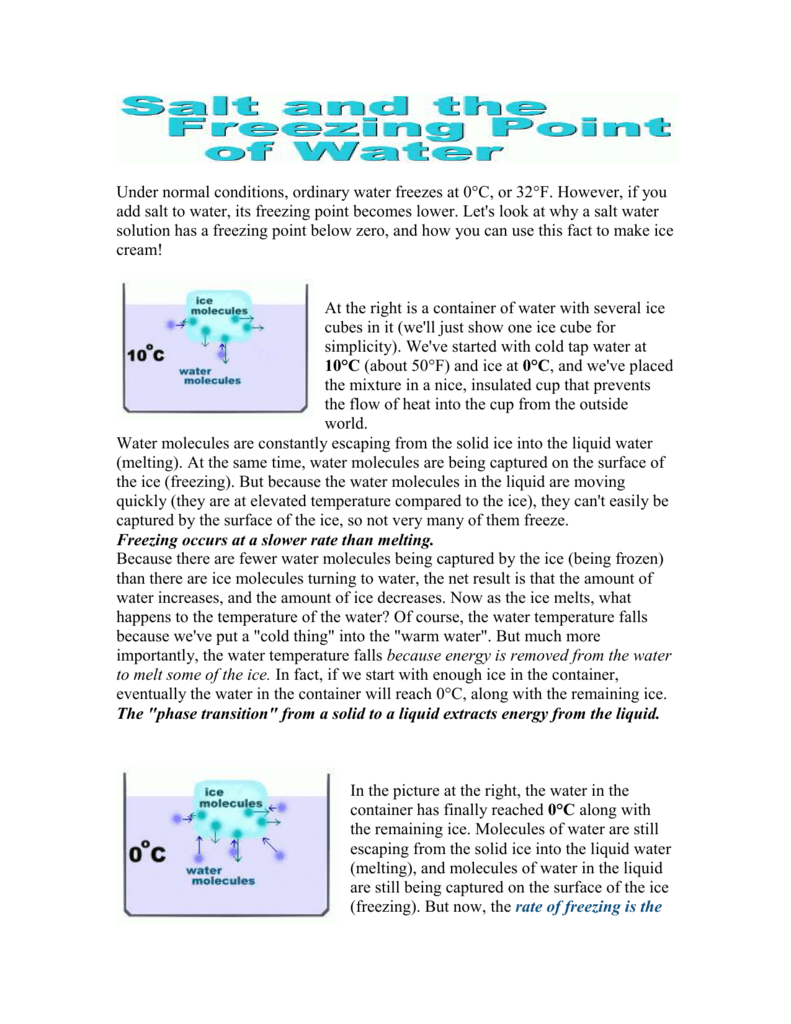
The water however can still melt the ice at that temperature which results in less ice on the roads. It causes ice that would have otherwise remained as a solid in sub-zero temperatures to turn to water. At 0C equilibrium is reached and the temperature cannot go any lower.
Thats because salt lowers the melting point of ice so any ice that remains in the solid state has to be colder than regular ice to still be solid. When salt is added to ice it lowers the melting point. Either can help reduce the freezing point so that the ice lasts longer.
When you add just ice to the ice cream maker the ice absorbs heat from the surrounding and starts melting. Magnesium chloride works down to 5F while calcium chloride works down to -20F. This is because salt is used to melt the ice and snow and keep it from refreezing.
Ice forms when the temperature of water reaches 32 degrees Fahrenheit 0 degrees Celsius and that includes ice on roadways. However this will only help if you follow a few other tips as well. Add Rock Salt to your Ice Chest.
When you add salt to an ice cube you end up with an ice cube whose temperature is above its melting point. In both cases the answer is based on the fact that adding salt to an ice water mixture in equilibrium lowers the freezing point or melting point of the equilibrium. Road salt works by lowering the freezing point of water via a process called freezing point depression.
Does salt make ice hotter. Why does the temperature get lower. When added to ice salt first dissolves in the film of liquid water that is always present on the surface thereby lowering its freezing point below the ices temperature.
Salt provides the solution. Does having a solution change the individual properties. Ice in contact with salty.
The effect is termed freezing point depression. If it gets warmer more ice becomes water. Magnesium chloride works down to 5F while calcium chloride works down to -20F.
This week Reactions is look at the science behind rock salt and how it melts ice. In this video I show you that salt NaCl does not actually melt ice despite our regular usage of the term melt I show you how confusing it is and then tal. This ice cube will do what any ice cube above its melting point will do.
As it melts it cools down since energy is being used to break bonds in the solid state. Similar to sugar salt affects how water freezes and effectively lowers the freezingmelting point of water. When salt is added to the ice bath usually rock salt in ice cream making it comes into contact with the thin layer of water on the surface of the melting ice.
Maintain Ice and Water Levels. When salt is added to ice what it does is that dissolves in a thin layer of the water that is present on the surface of the ice. This is why salt is added to ice on the roads in the winter.
Creating a saltwater slush and packing this around our ice cream base allows us to cool the base enough so that it starts to thicken and freeze before the ice melts completely. Were breaking down the chemistry that keeps the roads safe when bad weathe. Thus this layer has a lower freezing point than that of the pure water as the water changes into ice.
In other words the ice begins melting at a temperature lower than 0C. This principle is used to freeze cream to make ice cream. What does adding salt to ice do.
Thats a big difference. On the other hand if you put the same salt on ice at 15F the salt will be able to prevent melting ice from re-freezing. A 10-percent salt solution freezes at 20 degrees Fahrenheit -6 Celsius and a 20-percent solution freezes at 2 degrees Fahrenheit -16.
This salt water has a lower freezing point so the temperature of the ice bath can get even colder thus freezing the ice cream more quickly. On the other hand if you put the same salt on ice at 15F the salt will be able to prevent melting ice from re-freezing. When you add salt to ice which always has an outer film of water so its technically ice water the temperature can drop from freezing or 0 C to as low as -21 C.