Types and Examples Intermolecular Forces are the forces that exist between the molecules of a compound. Ionic Hydrogen bonding dipole-dipole Van der Waals dispersion forces.
Intermolecular forces are much weaker than intramolecular forces.

Types of intermolecular forces. Describe the types of intermolecular forces possible between atoms or molecules in condensed phases dispersion forces dipole-dipole attractions and hydrogen bonding Identify the types of intermolecular forces experienced by specific molecules based on their structures. These forces mediate the interactions between individual molecules of a substance. Intermolecular forces dispersion forces dipole-dipole interactions and hydrogen bonds are much weaker than intramolecular forces covalent bonds ionic bonds or metallic bonds.
Heres a closer look at these three intermolecular forces with examples of each type. What type of intermolecular force leads to high-density polythene. Intermolecular forces often abbreviated to IMF are the attractive and repulsive forces that arise between the molecules of a substance.
The three major types of intermolecular interactions are dipoledipole interactions London dispersion forces these two are often referred to collectively as van der Waals forces and hydrogen bonds. Measure of intermolecular force boiling point melting point DHvap DHfus DHsub 4. Within this set there are three main types of forces.
Intermolecular forces observed between atoms and molecules can be described phenomenologically as occurring between permanent and instantaneous dipoles as outlined above. Which is the strongest intermolecular force below Hydrogen bonding. There are many types of intermolecular forces IMFs.
Has no separation of charge so no positive or negative poles are formed. Solutions consist of a solventand solute. The attraction is primarily a result of the electrostatic forces.
The solvent then is a liquid phase molecular material that makes up most of the solution. Intermolecular forces are mainly of two types repulsive forces and attractive forces. Intramolecular forces are the forces that hold atoms together within a molecule.
Ionic bonds Hydrogen bonding Van der Waals dipole-dipole interactions Van der Waals dispersion forces. The intermolecular forces of attraction are also known as Van der Waals forces. Water is a good example of a solvent.
However there can be other causes of attraction between two or more constituents of a substance. Watch this video on YouTube Intramolecular forces or intra-molecular bonds are formed when the atoms of a particular molecule bind together or form a bond. Hence it is a polar molecule with dipole-dipole forces.
Intermolecular forces are the forces. Intramolecular Force that hold atoms together in a molecule Intermolecular are attractive forces between molecules 3. Intermolecular forces are responsible for most of the physical and chemical properties of matter.
There are three major types of intermolecular forces. What type of intermolecular force is so2. There are gas liquid and solid solutions but in this unit we are concerned with liquids.
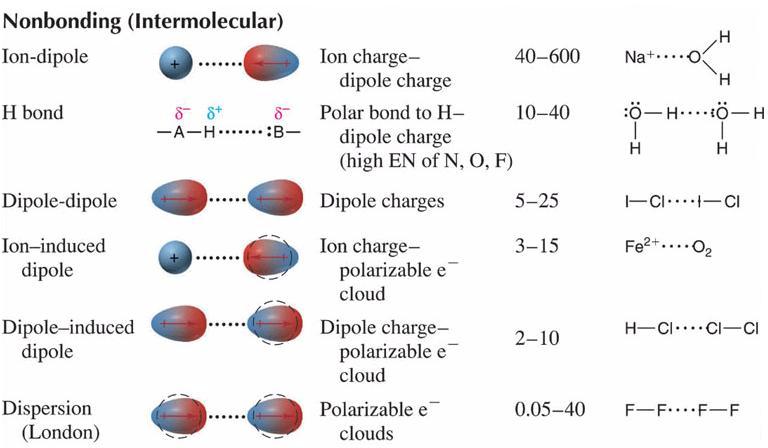
Van der Waals forces between closely packed linear polymeric chains are responsible for high-density polythene. The different types of intermolecular forces are hydrogen bond ion-dipole forces dipole-dipole forces ion-induced dipole forces dipole-induced dipole bond and dispersion forces. So now we can define the two forces.
Learn more by watching this informative video reviewing all the intermolecular forces The first of these is the set of Vander Waals forces which do not involve ions. Alternatively one may seek a fundamental unifying theory that is able to explain the various types of interactions such as hydrogen bonding van der Waals forces and dipoledipole interactions. Intermolecular forces are forces that exist between molecules.
Polymers - Some Important Polymers. The forces help to determine the physical properties of a molecule such as melting point boiling point density etc. The boxes represent the type of compound while the lines represent the type of force.
Types of intermolecular forces are. The types of intermolecular forces. What type of IMF is present in all substances regardless of polarity.
Figure of intermolecular attraction between two H-Cl molecules and intramolecular attraction within H-Cl molecule. The relative strength of the four intermolecular forces is. The last three forces dipole-dipole forces dipole-induced dipole forces and induced dipole forces are sometimes collectively known as van der Waals forces.
SO2 has a bent structure and has some net dipole moment. Advertisement Remove all ads. London dispersion force dipole-dipole interaction and ion-dipole interaction.
Types of Intermolecular Forces.