You may add more than one ball of sodium alginate to the calcium lactate bath at a time. Inhabitats DIY video showing how to make an edible water bottle using spherification.
 Ooho Will These Edible Blobs Replace Water Bottles Green Living Reset Org
Ooho Will These Edible Blobs Replace Water Bottles Green Living Reset Org
If you prefer something more flavorful you might enjoy a Japanese raindrop cake instead.

How to make edible water bottles. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Once the sodium alginate is full dissolved let the solution sit for 15-30 minutes to ensure there are no air bubbles. The Ooho is made using sodium alginate and calcium chloride using a formula developed by the designers.
See more ideas about edible water bottle edible water bottle. After those two are combined spoonfuls are put into a bath of calcium lactate which is a salt that can be. Use the hand mixer to make sure the sodium alginate is combined with the water.
Hand Mixer Ice Cube Trays Slotted Spoon A Small Bowl 2 Large Bowls A Whisk Mio Liquid Food Coloring 4 Cups of Water And an Additional 2 cups 1 tsp. Awater bottle you can eat. In a small bowl add 1 gram of sodium alginate to 1 cup of water.
Use a kitchen or a digital scale to measure out 1 gram of sodium alginate. An edible water bubble or bottle is water that has been solidified into a bubble-like shape. Dissolve Sodium Alginate in Water.
IN THIS VIDEO I WILL SHOW YOU HOW TO MAKE WATER BOTTLE WHICH U CAN EAT. Make an Edible Water Bottle. SORT OF A BUBBLEYOU NEED CALCIUM LACTATE-httpswwwamazoninUrban-Platter-Calcium.
The process combines drinking water with sodium alginate which comes from brown seaweed. Learn How to make a DIY edible liquid water bottle food you can eat. Set aside for 10-15 minutes until there are no air bubbles and solution is crystal clear.
Of Calcium Chloride More Water. Of Sodium Alginate 12 tsp. EDIBLE WATER BOTTLE NO PLASTIC HOW TO MAKE GIANT POPPING BOBA WATER BALLS jelly soft eating sounds DIY liquid water bo.
In this fun easy do it yourself science experiment tutorial youll be eating polymer bal. Perfect project for teens. Set a timer for 10 minutes minimum and remove the water bottles with a slotted spoon.
Plastic-free crystal clear healthy and FREE. The mixture will turn from a white liquid to a clear mixture. Sep 26 2017 - Explore mss board edible water bottle science project on Pinterest.
Let the mixture sit for about 15 minutes to remove any air bubbles. The raindrop cake itself is flavorless unless you sweeten it with vanilla sugar or drizzle sweet. Make edible water balloons or water bottles with this nifty DIY tutorial.
Making Edible Water Bubbles 1 Mix 1 gram of sodium alginate with 1 cup 240 mL of water. ASMR galaxy food galaxy dessert. It is made from water sodium alginate and calcium lactate.
How to Make Edible Water Bubbles The first step is to make the sodium alginate solution. Use the hand mixer or immersion blender to combine the two ingredients thoroughly. Blend 1 gram sodium alginate in 1 cup of water add food coloring if desired in a small bowl with an immersion blender.
Add one gram around ½ t of sodium alginate to the bowl containing one cup of drinking water. How would you like to have an edible water bottle.
Because of the shape the dipoles do not cancel each other out and the water molecule is polar. Water is considered a polar molecule because it has a perceptible electromagnetic field thats grouped around a positive and a negative pole like a magnet.
What Is A Polar Molecule Quora
The unequal sharing leads to polarity of water.

Explain why water is a polar molecule. On the other hand carbon dioxide is linear. So In this article I tried to cover the nonpolar nature of Carbon Tetrachloride moleculeCCl4. The bonds between hydrogen and oxygen are distributed so that the hydrogen atoms are both on one side of the oxygen atom rather than evenly spaced.
Water is a polar molecule due to the unequal sharing of electrons. The molecule is made up of two hydrogen atoms and one oxygen atom. Explain why the water molecule is a polar molecule but the carbon dioxide molecule is not polar even though both molecules contain polar bonds.
When solutes are added to water they may be affected by the charge distribution. Water H 2 O is a polar molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.
In the covalent bond between oxygen and hydrogen the oxygen atom attracts electrons a bit more strongly than the hydrogen atoms. The hydrogen atoms have one electron in each of them and the oxygen has 4 electron pairs. Two electron pairs of oxygen form covalent bonding with two electrons of hydrogen.
Oxygen is more electronegative than hydrogen and thus pulls the shared electrons in the covalent bond closer towards its nucleus. It means that there is an uneven distribution of electron density. Polarity of a Water Molecule.
The first thing to understand is. This forces most of the electrons to the side of the molecule where oxygen is present creating a highly negative area. The other side of the molecule becomes more positive due to the protons of the hydrogen atoms.
Water has two positively charged hydrogen atoms and one negatively charged oxygen atom bonded covalently in a single water molecule. In the figure below the net dipole is shown in blue and points upward. Polar simply means the electron density is not distributed evenly across a molecule.
That is it has one side that is positively charged and one side that is negatively charged. Water molecule is Polar due to its shape. Water is a bent molecule because of the two lone pairs on the central oxygen atom.
As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. This unequal sharing is due to oxygen being larger and getting the electron more than hydrogen. The oxygen side of the molecule has a slight negative charge while the side with the hydrogen atoms has a slight positive charge.
A water molecule because of its shape is a polar molecule. This is a result of the special way that the atoms of a water molecule are grouped. Water is made up of two hydrogen atoms both bonded to an oxygen atom.
Water H 2 O is polar because of the bent shape of the molecule. The water molecule as a whole has 10 protons and 10 electrons so it is neutral. The oxygen atom has 6.
In water this is caused by the comparatively higher electronegativity of the Oxygen you can think of this like Oxygen wants the electrons it shares in the covalent bond a lot more than the Hydrogen does so it keeps them closer but this is an oversimplification. The reason a water molecule is polar is due to its electron structure. Water is a bent molecule due to the lone pairs present in oxygen.
This is an example of polar covalent chemical bonding. However the C-Cl bond is a polar covalent bond but the four bonds cancel the polarity of each other and form a nonpolar CCl4 molecule. Water is a polar molecule because its oxygen is strongly electronegative and as such pulls the electron pair towards itself away from the two hydrogen atoms thus acquiring a slightly negative charge.
In a water molecule the oxygen atom and hydrogen atoms share electrons in covalent bonds but the sharing is not equal. The individual dipoles point from the H atoms toward the O atom. The bonds between the atoms are called covalent bonds because the atoms share electrons.
A molecule of water consists of two hydrogen atoms bonded to a single atom of oxygen. Water is a dipolar molecule because each atom has a dipole or partial charge. Water is a polar molecule because of its structure.
Water has a partial negative charge near the oxygen atom due the unshared. In carbon dioxide the carbon is flanked by the oxygen atoms OCO. The molecule of CCl4 is nonpolar in nature because of its symmetrical tetrahedral structure.
Groundwater often percolates through limestone where it picks up calcium and magnesium deposits. Hard water water that contains salts of calcium and magnesium principally as bicarbonates chlorides and sulfates.
 Hard Water Why You Need Soft Water Ne Water Softeners
Hard Water Why You Need Soft Water Ne Water Softeners
Hard water makes it harder to rinse soap plus it forms a curd or soap scum.

What is hard water. Hard water occurs under the natural condition when the water moves through rocks like limestone and chalk that consist of minerals like calcium carbonate and magnesium carbonate. Drinking hard water and beverages made using hard water can contribute to dietary requirements for calcium and magnesium. This is caused by the soap reacting with calcium to form soap scum.
Hard water is formed when water percolates through deposits of limestone chalk or gypsum which are largely made up of calcium and magnesium carbonates bicarbonates and sulfates. The calcium magnesium and other cations precipitate onto the resin surface. This means the water that comes out of your faucets is softened.
All kinds of minerals can end up being suspended in the water but the most common ones by far are calcium and magnesium. The difference between showering with hard water vs soft water is like night and day. In general hard water is not harmful for health.
In fact it may confer some benefits because it is rich in minerals and reduces the solubility of potentially toxic metal ions such as lead and copper. Hard water vs soft water is simply a comparison of water that has been treated for scale prevention and that which hasnt. Its a natural result of minerals like calcium and magnesium accumulating during the water cycle and it can happen with well water and even city water.
Water flows over the resin surface dissolving the sodium. Hard water is water that has high mineral content in contrast with soft water. A water softener is an appliance that works by exchanging the calcium and magnesium in hard water with the salt in sodium pellets.
Basically hard water is the result of minerals being dissolved in the liquid. Hard drinking water may have moderate. What does hard water contain.
You may be wondering what is in hard water and why it might be an issue in your home. The ion exchange resins are complex sodium salts. You may have felt the effects of hard water literally the last time you washed your hands.
Hard water is basically water containing a high mineral count. Iron oxides or iron carbonates can give a reddish brown colouration to hard water deposits. Hard water can even make your hair colorists job harder says Mara Roszak celebrity hairstylist and salon owner.
Hard water is a very common problem affecting water in more than 85 of the country. Drinking water can also contain trace minerals like iron which gets picked up from the soil lakes and rivers even older corroded plumbing. Before this process begins rainfall is initially what we describe as soft water which contains low to no mineral content.
A water softener system uses salt to create an electrical reaction in your water that pulls the hard minerals from the water. If water hardness is a factor in your area and a simple test will tell you a water softener is the most reliable way to ensure that your home is protected from damage by scale build-up. Hard water is high in dissolved minerals largely calcium and magnesium.
Signs of hard water include. Water with high mineral content is known as hard water. Hard water is water that has a high mineral content.
When the hair is difficult to work with this leads to more stress on the. High concentrations of minerals can be problematic however. Hard water can be softened have its minerals removed by treating it with lime or by passing it over an ion exchange resin.
Trace amounts of other minerals. It is a result of the dissolved minerals calcium magnesium and manganeseWith an increase in these minerals the following are seen 1. Soap is a less effective cleaner in hard water.
All water except for water that has been meticulously distilled will have some dissolved minerals since water is an excellent solvent and it readily combines with the substances it comes into contact with. Hard water contains a significant quantity of dissolved minerals such as calcium and magnesium. Hard water may offer health benefits as drinking water compared with soft water.
Ferrous iron may also be present. Hard water is water with a high dissolved mineral content while soft water has a relatively low concentrated of dissolved minerals. You may need to rinse your hands longer if the.
Hard water is therefore water that has been through this process and contains higher levels of minerals at the end. Hard water usually leaves a white chalky stain on the faucet which is an indicator that you have hard not soft water. Oxidized to the ferric form it appears as a reddish brown stain on washed fabrics and enameled surfaces.
Hard water is the water that contains a high amount of minerals like calcium magnesium and sometimes manganese and other cations. Feeling a film on your hands after washing them. What Is Hard Water.
The term hard water is used to describe a fresh water supply that contains relatively high amounts of natural minerals including calcium and magnesium. The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. If youre watching your salt intake and dont want to consume it in your drinking water you can filter the faucet have your water softener bypass the kitchen sink or purchase a softener that works with.
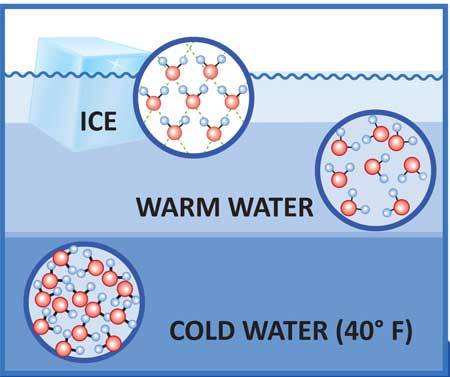
As such ice occupies about 9 more space than water so a liter of ice weighs not exactly liter water. Because water is a special case.
 Why Does Ice Float Guernseydonkey Com
Why Does Ice Float Guernseydonkey Com
Why Does Ice Float.
/why-does-ice-float-604304_final-5c891ca046e0fb00010f1193.png)
Why ice floats on water. The ice is lighter than the water because the density of ice is lesser than that of water. Water freezes at zero degree centigrade to form ice and it expands. It is common for us to observe ice cubes floating when placed in a glass of water and icebergs floating on the surface seas and oceans.
At zero degrees ie the temperature at which water turns into ice the density of water is actually quite low. Since the ice is slightly less dense than water the mass of the water it floats on is greater than its own resulting in buoyancy. This allows life to flourish even in the coldest climates on Earth.
This produces a crystal lattice commonly known as ice. Why Does Ice Float on Water. The density of the solid phase is generally larger than that of its liquid counterpart.
These bondings leave behind the larger amount of empty space due to which density decreases. But ice or water in its solid state floats in liquid water for having a lesser density. The heavier water displaces the lighter ice so ice floats to the top.
This is mainly because of the hydrogen bonding present in the molecules of water. When ice melts the stable crystal structure collapses and is suddenly denser. Typically solids are denser than liquids of the same substance such as solid lead but that is not the case with water and ice.
Water is filled in empty spaces available between rocks. As soon as water freezes the volume is increased which produces strong force e to which pipes burst. A water molecule is a V-shaped molecule made up of one oxygen atom in the centre with a hydrogen atom on each side.
Ice floats in water in similar manner. This also explains why youll never see ice forming at the bottom of bodies of water but at their surface instead. It turns out that ice has a lower density than water and any object that has a lower density than the liquid form on which its kept in this case water will be able to float.
Ice floats on water because the density of ice is less than that of water. Ice floats on Water. Since ice floats it can form on the surfaces of bodies of water creating a layer that provides insulation.
Ice floats on water because it is less dense than water. However this is a peculiar behavior as solid matter usually sinks in liquid. Density of solid becomes less when it expands.
Additionally the frozen layer provides a solid habitat for some animals such as. Ice floats in water because it less dense than water thanks to a special kind of chemical bonding known as hydrogen bonding. Solid ice and liquid water are the phases of the same substance.
Ice floats because it is about 9 less dense than liquid water. Waters unusual behavior can only be explained by its polarity and tendency to hydrogen bond which causes expansion upon freezing. This happens because of a chemical bonding called hydrogen bonding.
Therefore ice is less dense that water and floats. However in the case of water ice is less dense compared to water and this is the reason why ice floats. - Because ice floats the upper frozen layer insulates the liquid water below preventing the water below from freezing- supporting aquatic life.
This produces a crystal lattice which is usually known as ice. In other words ice takes up about 9 more space than water so a liter of ice weighs less than liter water. Consider what would happen if ponds and other bodies of water accumulated ice at the bottom.
The Archimedes Principle states that for an object to float on water it has to displace an equal amount of water. In Finland this property is utilized in breaking rocks. In common language we can say that ice floats on water because it is light as compared to water or after solidifying ice.
Ice floats since it has about 9 less dense than liquid water. Bursting of water pipes in cold regions is due to the volume of ice being greater than that of water. The unique behavior of water has been a discussion in many fora since both ice and water are water.
If solids are denser than liquids why does ice float on water. Why Does Ice Float on Water. The heavier water displaces the lighter ice so ice floats to the top of the water.
The molecules in water are affected by a phenomenon known as hydrogen bonding. A solid floats in water when the density of the displaced water is greater than that of the solid itself. As water warms past 4 C it gains energy and the molecules move.
Describe why this property of water is important.
An average tree needs about 16 cups 3800 mL of water per day. Lemonade contains water lemon juice and sugar which are all helpful to keep your Christmas tree healthy.
 Leaving Your Christmas Tree For A Couple Days Set Up A Reservoir Of Water With A Siphoning Tube To Your Tree Stand Lifeprotips
Leaving Your Christmas Tree For A Couple Days Set Up A Reservoir Of Water With A Siphoning Tube To Your Tree Stand Lifeprotips
Lots of additives have been suggested to keep your tree fresh including commercial tree preservatives molasses sugar bleach soft drinks aspirin honey and fertilizer.

What to put in christmas tree water. Aspirin may help ward off mold but again this is very unlikely for a tree to deal with. As a rule of thumb a Christmas tree needs 4 cups 950 mL of water for every 1 in 25 cm of its diameter. Its normal for your tree to take in a large amount of water one day and just a little the next.
Using a tree saw make a straight cut along the bottom of the trunktaking at least one inch off the original harvest cutand immediately place the new cut in water. According to the United States Department of Agricultures Forest Service these are the ingredients in a magic mixture for. Christmas tree experts dont recommend it either.
Devices are available that help maintain a constant water level in the stand. Water He recommends putting your tree in water as soon as you bring it home. Not only is this trick likely to extend the life of your tree but it can also boost the longevity of its scent too.
The glucose in sugar helps to maintain cell structure which in turn prevents needle. From there provide a quart of water for every inch of tree diameter. Sugar feeds the tree bleach acts as an antifungal and the lemon juice makes the water more acidic helping the tree to absorb it water better.
Most Christmas tree stands will hold about a gallon of water and they should be refilled daily. As a general rule stands should provide 1 quart of water per inch of stem diameter. So the next time someone tells you to add ketchup or something more bizarre to your Christmas tree stand dont believe it.
Gary Chastagner of Washington State University. Fresh tree fresh cut and fresh water are essential in keeping your real Christmas tree fresh and hydrated throughout the holiday season. Christmas tree stand Many stands are designed hold extra water as a reservoir for thirsty trees.
Christmas trees dont need to produce more branches or needles. A Fresh Cut-Fresh Cut Christmas Tree A fresh cut right before you put your Christmas tree in the stand is recommended. 8oz Karo Syrup clear the sugar feeds the tree 2 pinches of Epsom Salts magnesium sulfates make the needles green.
While its not a common problem its possible that contaminants andor bacteria in the water can lead to a foul odor. If its been more than three hours since you cut your tree down youll need to make another fresh cut immediately before you put the tree up to allow it to absorb water again. Need a Great Base.
Be sure to check and replenish your Christmas trees water every day. This will also help reduce the risk of fire. Check your stand to ensure its deep enough to hold all the water your tree needs.
Other Additives to Christmas Tree Water. This can also be used with flowers. A live Christmas tree drinks 1 quart of water per 1 inch of trunk diameter.
I would say it isnt worth adding. Hot water bleach corn syrup Epsom salt borax chelated iron. Same thing with your Christmas tree says Abbott.
This may seem like a given but dont forget to water your tree. But the cut tree slowly dries out and we want to preserve them as long as possible. To display the trees indoors use a stand with an adequate water holding capacity for the tree.
Use a stand that fits your tree. You will need to refresh your Christmas tree and open up the clogged cells so the tree can deliver appropriate moisture to the foliage. Keeping water in your Christmas tree stand helps keep your tree looking fresh and green throughout the holiday season as well as preventing dry needles from dropping off on your carpet.
So a tree with a 4-inch-diameter trunk will soak up a gallon of water every day. As a general rule your tree stand should hold at least 1 quart of water per inch of diameter of the tree trunk. It doesnt have to be distilled water or mineral water or anything like that.
Its pretty desperate for water since its had its root system removed Ignore online tips about adding orange juice vodka glycerin hydrogen peroxide or aspirin to the water or spritzing branches with hairspray to extend the life of your tree. A Christmas tree typically absorbs about one quart of water for each inch of its diameter. In the words of Dr.
There is nothing quite like a real Christmas tree. Make a fresh inch-wide cut at the base of your Christmas tree trunk then position it in a sturdy tree. Your best bet is just plain tap water added to the Christmas tree stand.
Recipe for Christmas Tree Water 2oz Regular Clorox Bleach prevents algae etc. For a Christmas tree though there really isnt anything chemically that will help a tree stay alive longer. Spray bottle Its important to make sure the trunk receives ample water but you also need a spray bottle to make sure the branches and needles get consistent moisture.
Choose one sturdy enough to support whichever size tree youve chosen. Pour in the warm sugar water and allow the tree to drink it up completely which will likely happen within just a few hours then continue to water as normal.