In this science activity children will explore chemical reactions with baking soda and vinegar. Baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion.
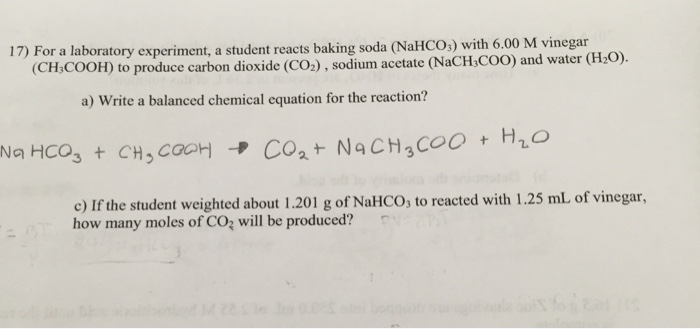
Baking Soda and Vinegar Reaction Equation Baking Soda Vinegar - Carbon Dioxide Water Sodium Ion Acetate Ion You can read more about the chemistry behind the reaction here.
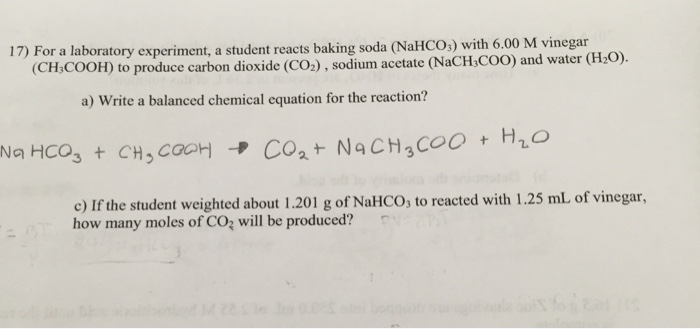
Baking soda and vinegar reaction equation. Refill the vinegar jars and pour in more baking soda and they are ready to go again. Baking Soda and Vinegar Rocket. The equation for the reaction is.
The first reaction is the acid -base reaction. The balanced chemical equation is. Frozen vinegar room temperature baking soda cold baking soda room temperature vinegar 1 tsp vinegar 12 tsp baking soda 1 tsp baking soda 12 tsp vinegar put the baking soda down first Any way lets go to step two.
If youre wondering if a chemical reaction is happening look for changes in. The baking soda and vinegar reaction is fairly short lived and depending on how many kids you have playing and how big they like to play you might run out of supplies pretty quickly. Vinegar or Acetic Acid has the chemical formula CH3COOH.
The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions. As you may be aware baking soda and vinegar are often used together in school science projects to create a bubbling fizzing reaction. This post contains affiliate links Exploring Chemical Reactions with Baking.
Balanced Chemical Equation for Baking Soda and Vinegar Reaction One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate one mole of water and one mole of carbon dioxide. Baking soda is a base also known as Sodium Bicarbonate and has the chemical formula NaHCO3. Acetic acid present in vinegar will readily react with baking soda sodium bicarbonate to form sodium acetate with the effervescence of carbon dioxide.
NaHCO 3 HC 2 H 3 O 2 NaC 2 H 3 O 2 H 2 O CO 2. Watch Queue Queue When baking soda sodium bicarbonate is combined with any type of vinegar dilute acetic acid or citrus juice citric acid carbon dioxide gas and sodium acetate form and produce a pretty spectacular bubbling reaction. This reaction creates sodium acetate water and carbon dioxide gas.
Not all chemical reactions need heat energy to make them happen. It is always fun to observe. When carbon dioxide is released we see it as a bubbling effect foam and fizz.
When bicarbonate of soda and vinegar are mixed the chemical reaction produces a gas. The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions. The baking soda and vinegar reaction is actually two separate reactions.
The reaction between baking soda and vinegar actually occurs in two steps but the overall process can be summarized by the following word equation. Baking soda vinegar carbon dioxide water sodium acetate NaHCO. This activity explores the popular baking soda and vinegar reaction which is a simple acid-base chemical reaction.
During this reaction the products are sodium acetate C2H3NaO2. Baking soda and vinegar chemical reaction Chemical reactions occur when 2 molecules interact to form new compounds or molecules. Baking soda is bicarbonate NaHCO3 and vinegar is acetic acid HCH3COO.
When the baking soda meets the vinegar there is a chemical reaction as carbon dioxide gas is created and fills the balloon causing it to inflate. The reaction proceeds in two steps. Fun investigation into how adding more vinegar changes the baking soda and vinegar reaction.
These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below. These two ingredients are so widely used in schools because they are a great example of a chemical reaction without the fear of children getting hurt in the process. The reaction proceeds in two steps.
We decided to add food coloring to the ingredient list to prompt even more discoveries. Created by LABScI at Stanford 4. Follow our Science for Kids Pinterest board.
CH3COOH NaHCO3 CH3COONa CO2 H2O. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Kids always love experimenting with baking soda and vinegar.
This clip is from. One of the products this reaction creates is carbon dioxide which makes the bubbles. The chemical equation for the overall reaction is.
When baking soda and vinegar mix together a chemical reaction is triggered.
On the otherhandthe vinegar is 6-10 Acetic acidThe formula for vinegar will be CH₃COOHThe IUPAC name for acetic acid is ethanoic acid. When baking soda and vinegar combine they react creating water and carbon dioxide.
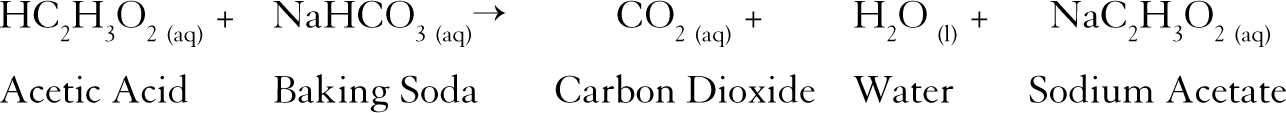 A Chemistry Puzzle To Be Solved Nsta
A Chemistry Puzzle To Be Solved Nsta
Little is known about the safety of taking both together so it may be safest to avoid.

Vinegar and baking soda formula. NaHCO 3 HC 2 H 3 O 2 NaC 2 H 3 O 2 H 2 O CO 2. Baking soda is a base also known as Sodium Bicarbonate and has the chemical formula NaHCO3. Measure out 1 cup 240 mL of rubbing alcohol 1 cup 240 mL of water and 1 tablespoon of white vinegar.
Baking soda and vinegar are best cleaners for your toilet. The baking soda and vinegar volcano is a fun chemistry project you can do to simulate a real volcanic eruption or as an example of an acid-base reactionThe chemical reaction between baking soda sodium bicarbonate and vinegar acetic acid produces carbon dioxide gas which forms bubbles in dishwashing detergent. Great addition to diaper pails.
The chemical equation for the reaction is Sodium bicarbonate and vinegar Sodium acetate and water and carbon dioxide NaHCO 3 CH 3 COOH CH 3 COO - Na H 2 O CO 2. Mix baking soda bicarbonate of soda and water to form a paste. The science behind baking soda bottle rockets.
Baking soda is essentially a base that reacts with other things that have acid content such as yogurt vinegar and buttermilk and releases carbon dioxide in the form of bubbles. Carbon dioxide bubbles through the liquid in the drain just as it does in a carbonated beverage. The reaction between baking soda and vinegar actually occurs in two steps but the overall process can be summarized by the following word equation.
Spread this paste all over the inside of your oven remove the racks avoiding the heating elements. Similar to yesterdays experiment we are working with an acid base chemical reaction in this project. In our case baking soda is sodium bicarbonate a base and vinegar is diluted acetic acid.
Brunette says to sprinkle 4 tablespoons of baking soda followed by two cups of vinegar. The ideal proportion is the following. Sprinkle baking soda on carpets let stand for 15-20 minutes and vacuum to remove odors.
A pinch of baking soda. For it to be truly effective it should be raw or unpasteurizedAs a result you will benefit more from all of its properties. Pour ½ cup of vinegar over sprinkled baking soda.
The baking soda and vinegar solution works in similar fashion to some commercial foaming drain cleaners but without the harsh chemicals. Try to get organic and high quality baking soda. Baking soda vigorously reacts with vinegar to produce carbon dioxide gas.
7Baking Soda and Vinegar Cleaning Solution for Toilet Bowl Cleaner. Vinegar or Acetic Acid has the chemical formula CH3COOH. Although both of these are leavening agents these release the trapped carbon dioxide in the batter or dough which subsequently makes it rise but baking soda.
Baking soda means sodium bicarbonate or sodium hydrogen carbonate. Baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion. For a DIY bathroom cleaner combine 1 23 cup of baking soda with 12 cup of dish soap in a bowl.
This activity explores the popular baking soda and vinegar reaction which is a simple acid-base chemical reaction. Baking soda is a base also known as Sodium Bicarbonate and has the chemical formula NaHCO3. Baking soda and apple cider vinegar may interact with medications and cause side effects of varying severity.
Leave an open box of baking soda in. Sprinkle baking soda on the dog and brush. Sprinkle ½ cup of baking soda in the toilet.
A glass of warm or hot water because cold water could be harmful to your liver. The balanced chemical equation is. The chemical formula for baking soda is NaHCO₃.
When they react to release the OH and H to become water they also release carbon dioxide. Sodium acetate is made of 1 sodium ion 2 carbon atoms 3 hydrogen atoms and 2 oxygen atoms. When the vinegar and baking soda react one of the bi-products of the reaction is the production of carbon dioxide gas.
This is how the baking soda and vinegar tip is supposed to work. Stir in 12 cup of water followed by 3 tablespoons of vinegar and continue mixing to combine the ingredients and get rid of any lumps. Once the bubbling stops flush the drain with boiling water.
Soak diapers in a solution of baking soda before washing to remove odors. Balanced Chemical Equation for Baking Soda and Vinegar Reaction One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate one mole of water and one mole of carbon dioxide. Spray the mixture onto glass mirrors ceramic tiles and chrome finishes then wipe with a paper towel or microfiber cloth.
Vinegar or Acetic Acid has the chemical formula CH3COOH. Here is a toilet bowl cleaning procedure. Let the bubbles subside for 10 minutes.
A tablespoon of organic apple cider vinegar. Scrub it with a toilet brush. This makes the reaction bubble and expand just like when you shake up a can of soda and open it.
Sprinkle baking soda in trashcans before and after adding trash bags. Pour them into a spray bottle. During this reaction the products are sodium acetate C2H3NaO2.
Heres how to clean your drain. During this reaction the products are sodium acetate C2H3NaO2. To easily apply this cleaner to any bathroom surface put it in a squirt bottle.