Two electrons in their outer shell. The elements have very similar properties.
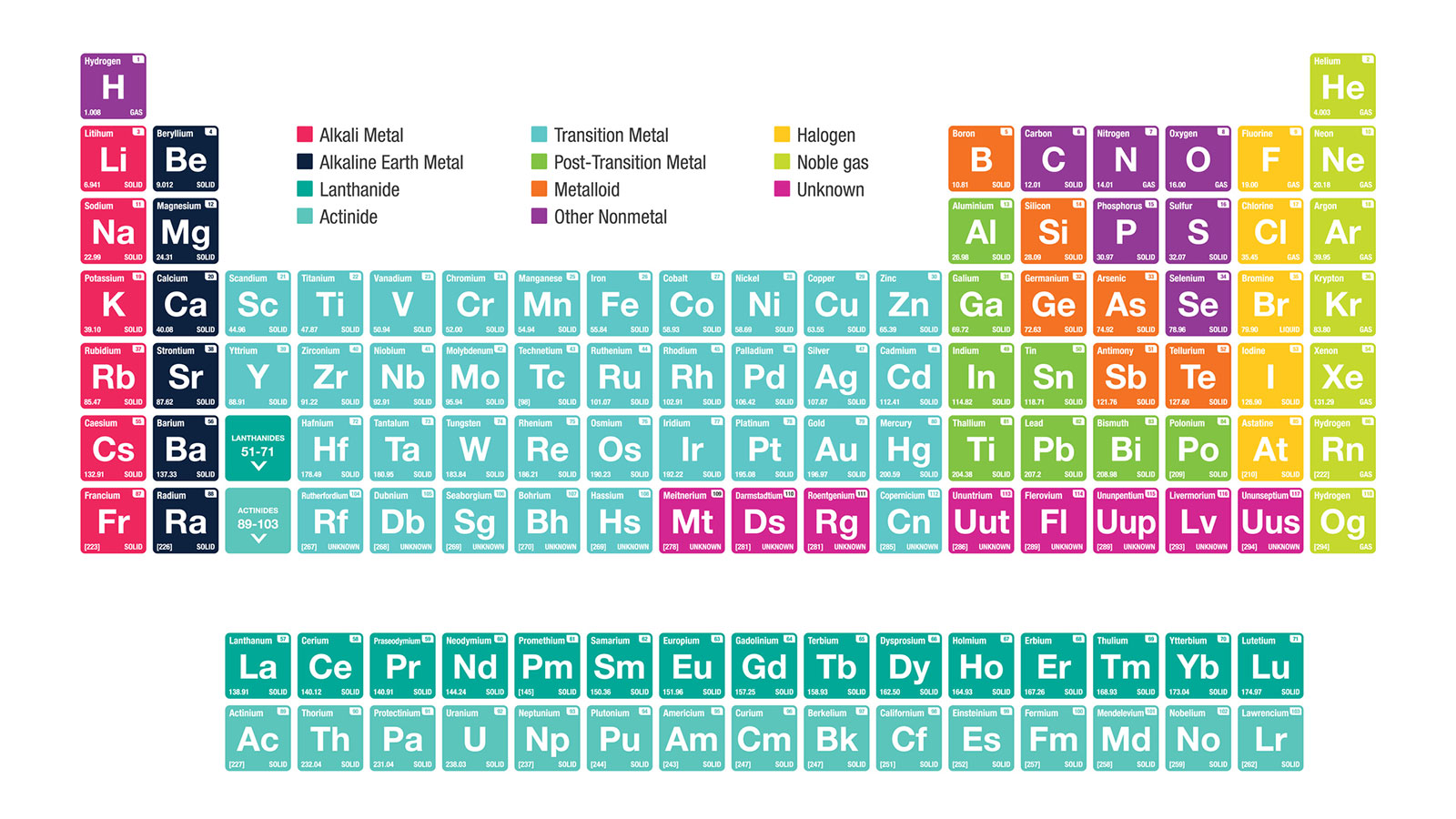
 Alkaline Earth Metals Periodic Table Groups
Alkaline Earth Metals Periodic Table Groups
The alkaline earth metals make up group 2 of the periodic table from Be through Ra.
Alkaline earth metals periodic table. Some characteristics of alkaline earth metals are. On this periodic table label the alkali metals alkaline earth metals halogens transition metals nonmetals and the noble gasses. Click here to buy a book photographic periodic table poster card deck or 3D print based on the images you see here.
Present in the earths crust but not in their basic form. Beryllium Be magnesium Mg calcium Ca strontium Sr barium Ba and radium Ra. As with all families these elements share traits.
The second column of elements from the left of the periodic table is known as the Group 2 or alkaline earth metals. Periodic Table Of Elements And PeriodicityLecture-2Some families in the periodic table. An oxidation number of 2 which makes them very reactive.
Not found freely in nature. The elements are beryllium Be magnesium Mg calcium Ca strontium Sr barium Ba and radium Ra. This group lies in the s-block of the periodic table.
The alkaline earth metals are a group of elements in the periodic table. 6 uses of alkaline earth metals in periodic table chemistry periodic table periodic table groups names and properties difference between alkali metals and Chem4kids Elements Periodic Table Alkaline Earth Metals6 Uses Of Alkaline Earth Metals In Daily Life Pounds Az ChemistryGroup 2 Elements Alkaline Earth Metals EmedicalprepPeriodic Table Of The Elements Alkaline Earth Metals Science. They are all shiny silvery-white somewhat reactive metals at standard temperature and pressure.
The alkaline earth metals are six chemical elements in group 2 of the periodic table. The elements have very similar properties. The members of the alkaline earth metals include.
The alkaline earth metals are in the second group of the periodic table. Location of the Alkaline Earths on the Periodic Table. One of the alkaline earth metals is Calcium the third most abundant metal after Iron and Aluminum.
Alkaline earth metals are the elements in main group 2 2A of the periodic table which is the second columnAll neutral atoms in this group will possess a condensed electron configuration that. They are sometimes referred to as the group 2 elements. What elements are alkaline earth metals.
The alkaline earths are the elements located in Group IIA of the periodic table. Alkali and Alkaline Earth Metals. The list of elements that are alkaline earth metals is short.
This is the second column of the table. To complete this quiz you will need access to a periodic table. TemplatePeriodic table alkaline earth metals Jump to navigation Jump to search.
This makes it irritating to try to keep samples of them. They are beryllium Be magnesium Mg calcium Ca strontium Sr barium Ba and radium Ra. This quiz game will make it easy for you to memorize all the alkaline earth metals.
Also label the number of valence electrons in columns 1213-18 2. Each alkaline earth metal atom has two valence electrons in its outermost shell s-orbital. The alkali metals make up group 1 of the Table and comprise Li through Fr.
The alkaline earth metals are six chemical elements in group 2 of the periodic table. There are 6 alkaline earth metals and they are part of Group 2 the second column of the periodic table. In order of increasing atomic number the six element names and symbols are.
It consists of beryllium magnesium calcium strontium barium and radium. They are ready to give up those two electrons in electrovalent ionic bonds. This group includes the elements lithium sodium potassium rubidium caesium and franciumEach of these elements has just one valence electron which means that they form.
They are beryllium Be magnesium Mg calcium Ca strontium Sr barium Ba and radium Ra. Distributed in rock structure. Alkaline earth metals are more stable than alkali metals.
Group 2A elements are called alkaline earth metals because they form oxides. Calculate the formula masses for the following compounds. Alkaline earth metals CAS group number US pattern A-B-A IIA old IUPAC number.
Each element in this family has two valence electrons which means that they will lose these electrons to form ions with a 2 charge. They are all in the second column of the periodic table. This gives alkaline earth metals the largest atomic radii in their periods.
Structurally they have in common er. The elements in group one of the periodic table with the exception of hydrogen - see below are known as the alkali metals because they form alkaline solutions when they react with water. The Alkali Earth Metals comprise the second column from the left.
Alkali metals Alkaline earth metals Halogens Noble gasesAlkali m. The alkaline-earth elements are highly metallic and are good conductors of electricity. The elements of the alkaline earth metals include beryllium magnesium calcium strontium barium and radium.
Alkaline-earth metal any of the six chemical elements that comprise Group 2 of the periodic table. Click the links or see below for more details on each. They have very similar behavior and characteristics.
They are reactive metals that tend to oxidize in air. While not as reactive as the alkali metals this family knows how to make bonds very easily. They are all shiny silvery-white somewhat reactive metals at standard temperature and pressure.
Originally the term alkaline earths referred only to the oxides of calcium strontium and barium. Hydrogen is group 1 but exhibits few characteristics of a metal and is often categorized with the nonmetals.
See the Periodic table below. List of Nonmetals Element Group There are 7 elements that belong to the nonmetals group.
Non-metals are further classified into the following groups.
:max_bytes(150000):strip_icc()/nonmetals-56a12d975f9b58b7d0bccfd3.png)
List of non metals. Gunmetal an alloy made from a mixture of copper tin and zinc Steel an alloy made from a mixture of iron and carbon. All of which are the basic building blocks of organic compounds. The nonmetal element group consists of hydrogen carbon nitrogen oxygen phosphorus sulfur and selenium.
List of Non-Metals on the Periodic Table. At room temperature many nonmetals hydrogen nitrogen oxygen fluorine chlorine and the noble gases are gases. In our day to day life we encounter so many useful metals and non-metals.
Metals and Nonmetals are an important part of our lives. We all know how useful metals and nonmetals are. The nonmetals The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc.
Find Our Periodic Table Labeled. We cant survive without nonmetals like oxygen and also without the existence of metals. The nonmetal element group is a subset of these elements.
The basic structure of society is built with the matter around us. Examples of some common alloys are detailed in the following list. Every one of you must be familiar with the materials like coal iron copper and many more.
The periodic table is organized in families and periods. These all metals and non-metals are studied in detail and accordingly put to use in daily activities. List Three Properties of Non-Metals.
The following are the general properties of non-metals. Hydrogen sometimes considered an alkali metal. Nonmetals in the gaseous state exist as single atoms or diatomic molecules.
List of Non-Metals and Their Symbols. Diamond is an allotrope of carbon and is the hardest natural substance known and has very high melting and boiling point. 1Oxygen is a non-metal which is used by plants and animals for breathingIt is essential for maintaining our lifeIt is used in the process of burning of fuels in homesfactories and transport vehicles.
Examples of non-metals include carbon oxygen nitrogen and hydrogen. It can be solid liquid and in gaseous state. As a result the differences between metallic and nonmetallic properties are evident within each group even though all members of each group have the same valence electron configuration.
The line that divides metals from nonmetals in the periodic table crosses the p block diagonally. So it is an exception in the case of nonmetals. Metals In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to.
This is because nonmetals differ widely in interatomic and intermolecular bonding strengths. The remaining nonmetals are solids. One useful way is by metals nonmetals and metalloids.
One nonmetal bromine is a liquid. During displacement reactions a more active nonmetal displaces a less active nonmetal from a compound. In the periodic table they are represented under Polyatomic non-metal Diatomic non-metal Noble gases.
This is the list of non-metals in the periodic table. They are the bad conductor of heat and electricity. Definition of reactivity of nonmetals Nonmetal activity series is a list in which nonmetals are arranged in the decreasing order of their reactivity.
In chemistry a nonmetal or non-metal is a chemical element that mostly lacks the characteristics of a metalPhysically a nonmetal tends to have a relatively low melting point boiling point and densityA nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivityChemically nonmetals tend to have relatively high ionization energy. The exception is hydrogen which behaves as a nonmetal at room temperature and pressure and is found on the upper left corner of the periodic table. Non-Metal dopants are carbon nitrogen fluorine sulphur and iodine.
Similar to metals we can also arrange nonmetals in terms of their reactivity. Nonmetals include the nonmetal group the halogens and the noble gases. Most of the non-metals are gasses.
Compared to metals nonmetals display a highly variable range of properties in terms of their atomic and chemical behavior. Using the periodic table you can classify the elements in many ways. Several of the other properties of non-metals result from their atomic sizes.
Carbon phosphorus sulfur selenium and iodine are the solid non-metals. The atoms of non-metals tend to be smaller than those of metals. Also graphite another allotrope of carbon is a good conductor of electricity which is another exception.
The nonmetals are in a minority on the periodic table mostly located on the right-hand side of the periodic table. Carbon is a nonmetal that can exist in different forms called allotropes. These elements have similar chemical properties that differ from the elements considered metals.
Alloys made of mixtures of at least one metal with either other metals or with non-metals. List of Metals - Alloys What are alloys. Question 7 State few uses of non-metals.
The alkali metals are lithium sodium potassium rubidium cesium and francium. In the periodic table the alkali metals are a group or column containing the chemical elements such as lithium Li sodium Na rubidium Rb potassium K francium Fr and Caesium Cs.
 Alkali Metals Facts About The Elements On The First Column Of The Periodic Table Howstuffworks
Alkali Metals Facts About The Elements On The First Column Of The Periodic Table Howstuffworks
This group includes the elements lithium sodium potassium rubidium caesium and franciumEach of these elements has just one valence electron which means that they form.

Alkali metals periodic table. The only element in the first column that is not usually considered an alkali metal is hydrogen. Alkali metals are found in which group of the periodic table. The alkali metals have the high thermal and electrical conductivity lustrous ductile and malleable that are characteristic of metals.
The alkali metals are lithium sodium potassium rubidium cesium and francium. This video below explains more about alkali metals or group 1 metals on the periodic table. This makes it easier for an atom to lose an electron from its outer shell.
Preview this quiz on Quizizz. Lithium Li sodium Na potassium K rubidium Rb caesium Cs francium Fr Properties of alkali metals. This is because the outer shell gets further away from the positive attraction of the nucleus.
The Periodic Table - the Alkali Metals. The alkali metals are so called because reaction with water forms alkalies ie strong bases capable of neutralizing acids. This common electron setup.
The metals whose oxides make up the alkaline earths then came to be known as the alkaline-earth metals and have been classified in Group 2 IIa of the periodic table ever since Russian chemist Dmitry Mendeleyev proposed his first table in 1869. Kids Learning Tube Learn about the Periodic Table of elements and the group alkali metals with this fun educational music video for children and parents. 19 Number of Neutrons.
Alkali metals are found in which group of the periodic table. When looking for families the first one you will find is the alkali metal family of elements. You should remember that there is a separate group called the alkaline earth metals in Group Two.
This group lies in the s-block of the periodic table as all alkali metals have their peripheral electron in an s-orbital. Francium is so radioactive and short-lived that nobody has ever seen a lump of it. The reaction is very vigorous and can sometimes result in explosions.
390 Number of ProtonsElectrons. 10th - 11th grade. Play this game to review Periodic Table.
Sir Humphrey Davy Uses. And so if this video will be talking about Group One which are the alkali metals and so alkaline metals are basically medals in Group one excluding hydrogen. BamlouGetty Images There are alkali metals all around you right now.
They all go on earth metals which are group too. They are all in the first column of the periodic table. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right.
General properties of alkali metals. They are also known as the alkaline metals. Alkali metals become more reactive as you go down the group in the periodic table.
This page introduces the Alkali Metals in Group 1 of the Periodic Table. The alkali metals are the elements located in Group IA of the periodic table. Alkali metal any of the six chemical elements that make up Group 1 Ia of the periodic table namely lithium Li sodium Na potassium K rubidium Rb cesium Cs and francium Fr.
And we also have our transition metals which is in the D block of the periodic table as well as the Atlanta nods and the active. It is the first group of s-block Despite the presence of hydrogen at the top of the group 1A It is not one of the alkali metals but it is one of the nonmetals because it has a small atomic size and it is a gas. Lets go to the left side of the periodic table.
Alkali metals make up six different elements found in the first column of the periodic table. The elements in group one of the periodic table with the exception of hydrogen - see below are known as the alkali metals because they form alkaline solutions when they react with water. Hydrogen and the alkali metals make up the group 1 elements of the periodic table.
We can however predict what its properties might. Alkali metals group is located on the maximum left side of the modern periodic table. The alkali metals are a group of elements in the periodic table.
Alkali and Alkaline Earth Metals. Sodium is found in table salt lithium in your phone battery and potassium in your bananas. As with the alkali metals of Group 1 Ia the atoms of the alkaline-earth metals easily lose.
The alkali metals are on the left column of the periodic table highlighted in hot pink. The alkali metals are among the most electropositive elements on the periodic table and thus tend to bond ionically to the most electronegative elements on the periodic table the halogens fluorine chlorine bromine iodine and astatine forming salts known as the alkali metal halides.
