The relative error of the measurement is 2 mph 60 mph 0033 or 33. The only trick here is that the volume isnt in liters.
 How To S Wiki 88 How To Calculate Percent Error Equation
How To S Wiki 88 How To Calculate Percent Error Equation
Chemists have to be concerned with just how completely their reactants react to form products.
How to find percent error chemistry. That is what percent they are. In some fields percent error is always expressed as a positive number. So a typical row would be name.
While this effect is not noticeable in everyday experience it becomes apparent at speeds approaching the speed of light. Most quantitative labs labs involving numerical measurements have a student calculate his or her percent error. How to calculate percentage error in chemistry.
Subtract the experimental value from the actual value and take its absolute value. The absolute value of the error divided by the accepted value and multiplied by 100. If you use your 15K extrapolation the percent error will always be 100 no matter what the error actually was.
To calculate the percent error you take the difference between a value collected in an experiment and a known or exact value. The absolute error of his speedometer is 62 mph - 60 mph 2 mph. In calculating percent error We need to get the experimental value and the value which you are aiming at which is the actual value first.
As you noticed the percent-error formula needs you to use a value in the denominator. Step 1 Solve Find Percentage Error Chemistry. Relative Error Error Known Value Relative Error - 029 g 580 grams Relative Error - 0050 Error Relative Error x 100 Error - 0050 x 100 Error - 50 Cite this Article.
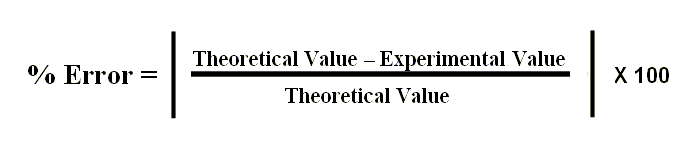
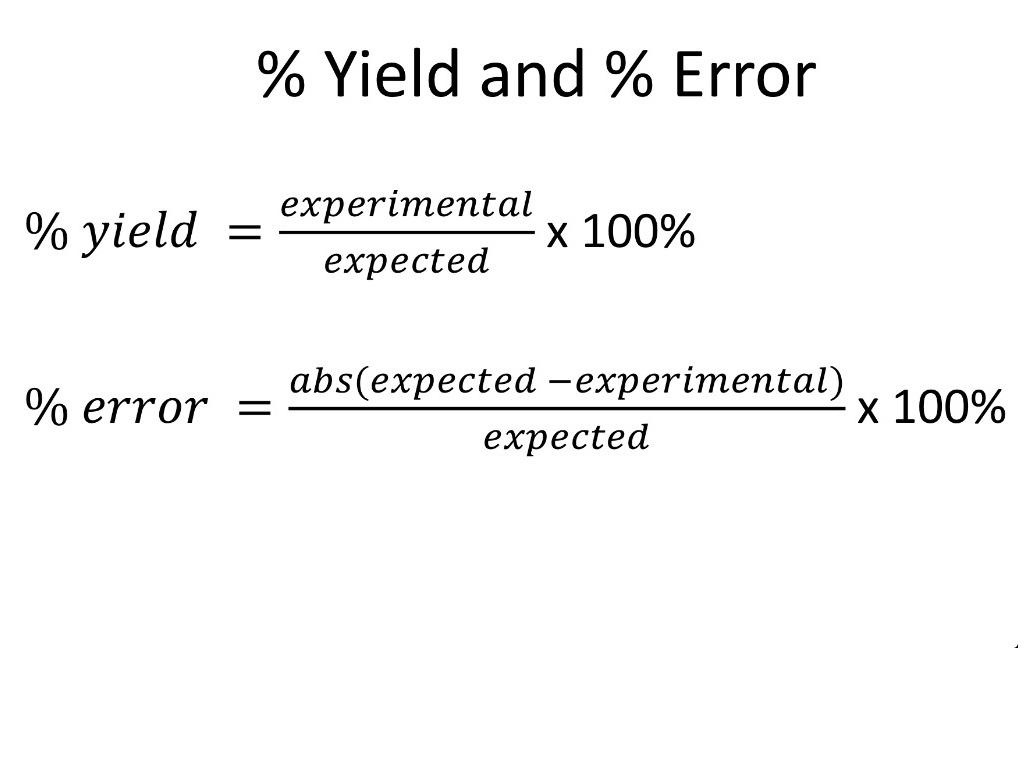
The formula to calculate Percent Error is. In such cases I would look at the percent error with respect to your data. Percent error percentage error is the difference between an experimental and theoretical value divided by the theoretical value multiplied by 100 to give a percent.
Divide the raw error by the actual value and multiply the relative error by 100 to get the percent error. The purpose of determining the percent error is to ascertain the difference between exactstandard values and experimental values. This formula is similar to percentage change.
Relative Error Absolute Error Known Value For example a drivers speedometer says his car is going 60 miles per hour mph when its actually going 62 mph. The experimental value is your calculated value and the theoretical value is your known value. Percentage Error E T T 100.
It doesnt matter what the nature of the compound is ie. You can calculate the percent recovery of such a procedure using the starting and ending weights of the chemical. Suppose you did an experiment to measure the boiling point of water and your results average to 1015C.
Would you like to safely and quickly eliminate Percentage Error. For example how to calculate the percentage error. Percentage Error Approximate Value Exact Value Exact Value 100.
Formula for Percent Error. A percentage very close to zero means you are very close to your targeted value which is good. Percent error is most frequently used in chemistry and the other physical sciences.
Is Find Percentage Error Chemistry appearing. In others it is correct to have either a positive or negative value. Time slows down for objects in motion.
The formula is given by. Where E is the experimental value and T is the theoretical value. If you use 0K in the denominator you get a divide by zero.