1 for cables with a service temperature of max. The carrying capacity is different for each species in a habitat because of that species particular food shelter and social requirements.
Selection on the basis of certain criteria types of tourist destinations types of environmental problems etc of case studies either sites where Tourism Carrying Capacity could be appropriate as a tool for managing pressures from tourism development or sites where TCC has been already implemented and detailed description of them.

What is carrying capacity. Capacity means the ability or the power to contain or producing the maximum output. The carrying capacity of an environment is the maximum population size of a biological species that can be sustained by that specific environment given the food habitat water and other resources available. The main aim of tourism carrying capacity is to prevent the over-utilization of the destination by identifying the maximum number of people who can visit an area.
Carrying capacity definition the maximum equilibrium number of organisms of a particular species that can be supported indefinitely in a given environment. Dengan kata lain carrying capacity dapat disebut juga sebagai kemampuan lingkungan ekosistem dalam mendukung kehidupan semua makhluk yang ada di dalamnya secara berkelanjutan. In other words a populations carrying capacity is the size at which a population can no longer grow due to lack of supporting resources.
Carrying Capacity 15 x Strength Score This means a creature that has a Strength of 10 can carry 150 pounds. Carrying capacity the average population density or population size of a species below which its numbers tend to increase and above which its numbers tend to decrease because of shortages of resources. It assesses the power of the Earth to sustain the maximum number of species without causing any damage to the ecosystem.
Although it can be. In ecological terms carrying capacity is defined as the maximum number of a species that can sustainably live in a given area. Strength 8 can still carry 120 pounds.
Thus Carrying Capacity means the ability to sustain up to a certain limit or scope. In population ecology carrying capacity is defined as the environments maximal load which is different from the concept of population equilibrium which may be far below an. This uses data that is about 8 years old but it is useful if you explain this to your students from the start.
Carrying capacity is a concept that has been widely applied in tourism and recreation studies since the 1960s although some authors trace its emergence to as far back as 1930. However it is very difficult for ecologists to calculate human carrying capacity. 70C at the conductor The maximum current-carrying capacity acc.
Carrying capacity is not fixed. Carrying capacity is not just a scientific concept or formula of obtaining a number beyond which development should cease but a process where the eventual limits must be considered as guidance. Konsep ini diperkenalkan oleh Thomas Robert Malthus yang di.
The carrying capacity for any given area is not fixed. Data are always changing on carrying capacity estimates. Carrying capacity refers to the number of individuals who can be supported in a given area within natural resource limits and without degrading the natural social cultural and economic environment for present and future generations.
Carrying capacity atau daya dukung adalah jumlah maksimum individu yang dapat didukung atau dilayani oleh sumber daya yang ada di dalam suatu ekosistem. An ecosystems carrying capacity for a particular species may be influenced by many factors such as the ability to regenerate the food water atmosphere or other necessities that populations need to survive. Basic carrying capacity presentation with key definitions and examples.
This likely varies over time and depends on environmental factors resources and the presence of predators disease agents and competitors over time. Carrying capacity is the maximum number of a species an environment can support indefinitely. In tourism the Carrying capacity is described as the most number of tourists that are accepted at a place without harming the physical atmosphere.
Carrying means to support or sustain. Humans are a complex species. Every species has a carrying capacity even humans.
To VDE 0891 part 1 point 7 has to be considered for the application of insulated cables in telecommunication systems and data processing units. Carrying capacity is the maximum number density or biomass of a population that a specific area can support sustainably. They should be carefully assessed and monitored complemented with other standards etc.
Carrying capacity is the number of organisms that an ecosystem can sustainably support.
The volumetric heat capacity measures the heat capacity per. The SI unit of heat capacity is joule per kelvin.
The subscript p indicates that the heat capacity and specific heat capacity apply when the heat is added or removed at constant pressure.
Define specific heat capacity. The measurement techniques of the specific heat capacity and the coefficient of thermal conductivity are shown. The heat capacity in calories per gram is called specific heat. We are giving a detailed and clear sheet on all Physics Notes that are very useful to understand the Basic Physics Concepts.
Specific heat capacity noun the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Where represents the amount of heat needed to uniformly raise the temperature of the sample by a small increment. Therefore the ratio between Cp and Cv is the specific heat ratio.
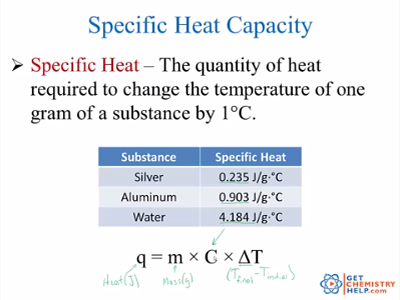
It is an intensive property as it is independent of the quantity or size of the matter. The heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Specific heat capacity is a measure of the amount of heat necessary to raise the temperature of one gram of a pure substance by one degree K.
Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The specific heat capacity of a substance usually denoted by is the heat capacity of a sample of the substance divided by the mass of the sample. The amount of heat required to raise the temperature of unit mass of the substance through 1C is called its specific heat.
C p for constant pressure Also called. The specific heat capacity of a material is a physical property. It is the ratio of two specific heat capacities Cp and Cv is given by.
The molar specific heat capacity of a substance is nothing but the amount of heat you need to provide to raise the temperature of one gram molecule of the substance through one degree centigrade. It is denoted by C. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass.
Specific heat capacity in British English. The main difference between specific heat and heat capacity is that specific heat is the amount of energy needed to raise the temperature of a given sample by 1 K while heat capacity is the amount of energy needed to raise the temperature of 1 kg of substance by 1 K. Heat capacity ratio of heat absorbed by a material to the temperature change.
The specific heat capacity of water is 4200 Joules per kilogram per degree Celsius JkgC. Learn more about it in this article. This is because of the reason that we defined unit of heat calorie by making use of water.
It is also an example of an extensive property since its value is proportional to the size of the system being examined. Specific heat capacity derivation and definition The specific heat capacity indicates how much heat must be absorbed by a substance of mass 1 kg in order to increase its temperature by 1 K 1 C. This means that it takes 4200 J to raise the temperature of 1 kg of water by 1C.
The Heat Capacity at Constant Pressure Cp Heat capacity at Constant VolumeCv The isentropic expansion factor is another name for heat capacity ratio that is also denoted for an ideal gas by g gamma. Usually measured in joules per kelvin per kilogram. Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to a given mass of a material to produce a unit change in its temperature.
Definition Units Types Thermometry and Calorimetry. The corresponding intensive property is the specific heat capacity. Scientists needed a quantity that has no dependence on the quantity or size of matter under consideration for thermodynamic studies this made them define specific heat capacity.
Usually measured in joules per kelvin per kilogram Symbol. What is Specific Heat Capacity. Specific heat capacity synonyms specific heat capacity pronunciation specific heat capacity translation English dictionary definition of specific heat capacity.
2014 SPECIFIC HEAT CAPACITY AND THERMAL CONDUCTIVITY OF HEAT STORAGE MATERIALS BASED ON PARAFFIN BROWN-COAL WAX AND POLYETHYLENE WAX Problems of the Regional. Specific Heat Capacity Definition. Specific heat of water is taken to be 1.
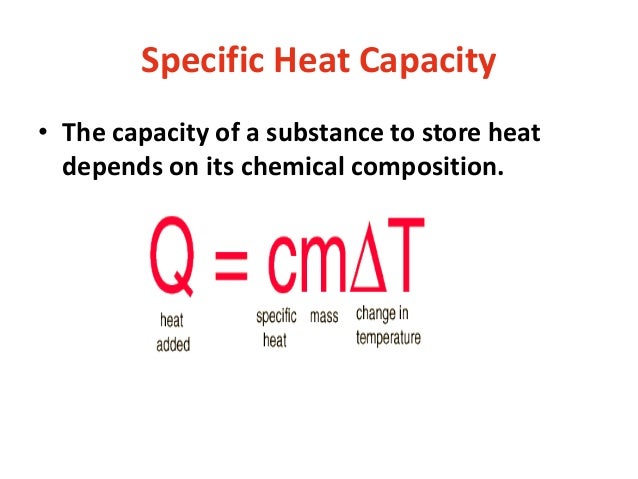
Cp Q mDT. Define specific heat capacity. What is Heat Capacity.
Like the heat capacity of an object the specific heat of a substance may vary sometimes substantially. It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. Cp for constant pressure.
Specific Heat Capacity The heat capacity of a substance per unit mass is called the specific heat capacity cp of the substance. Snezhkin Yu Mykhailyk V Korinchevska T Vorobiev L Dekusha L. Heat capacity is an extensive property.
N the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Specific heat capacity for any substance or matter can be defined as the amount of heat energy required to raise the temperature of a unit mass of that substance by one degree Celsius.
Specific heat is a particular type of heat capacity. Specific heat capacity definition.
 Specific Heat Capacity Spm Physics Form 4 Form 5 Revision Notes
Specific Heat Capacity Spm Physics Form 4 Form 5 Revision Notes
Like the heat capacity of an object the specific heat of a substance may vary sometimes substantially.
Specific heat capacity definition. Varying ranges of specific heat values are seen for substances depending on the extent to which they absorb heat. N the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Specific Heat Capacity Unit.
Heat capacity is an extensive property. C p for constant pressure Also called. Heat Capacity is the amount of heat required to increase the temperature of a substance to 1 degree Celsius C or 1 Kelvin whereas specific heat is the amount of heat required to increase the temperature of substance having mass 1kg or 1g by 1 degree Celsius C or 1 Kelvin.
Specific Heat Capacity Definition Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. We are giving a detailed and clear sheet on all Physics Notes that are very useful to understand the Basic Physics Concepts. Calculating thermal energy changes.
Hence its derived SI unit is J kg1 K1. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to a given mass of a material to produce a unit change in its temperature.
A specific heat capacity calculator is functioned to deliver the outcomes along with standardized units. Meaning pronunciation translations and examples. The SI unit of heat capacity is joule per kelvin.
The heat capacity of a substance per unit mass is called the specific heat capacity cp. Specific heat capacity noun the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Specific heat gives the heat capacity per kilogram of a substance.
The heat required to raise unit mass of a substance by unit temperature interval under. Specific heat capacity derivation and definition The specific heat capacity indicates how much heat must be absorbed by a substance of mass 1 kg in order to increase its temperature by 1 K 1 C. The amount of heat required to raise the temperature of unit mass of the substance through 1C is called its specific heat.
Definition of Specific heat capacity revealed that it is the amount of heat required to increase the temperature of 1 kilogram of any substance by 1 kelvin. It is also an example of an extensive property since its value is proportional to the size of the system being examined. Heat capacity C is the amount of energy needed to raise the temperature of a specific substance by 1 degree Celsius.
Usually measured in joules per kelvin per kilogram Symbol. Definition of Specific Heat and Heat Capacity Heat capacity gives the amount of energy required to raise the temperature of a given sample of a substance by 1 o C. Learn more about it in this article.
The volumetric heat capacity measures the heat capacity per. The specific heat capacity of a material is a physical property. Specific heat capacity is a measure of the energy required to raise the temperature of 1 kg of material by 1C.
The heat capacity in calories per gram is called specific heat. Heat capacity can also be viewed as the ratio of the amount of energy. The specific heat capacity of a material is the energy required to raise one kilogram kg of the material by one degree Celsius C.
Heat capacity ratio of heat absorbed by a material to the temperature change. The specific heat capacity of a substance usually denoted by is the heat capacity of a sample of the substance divided by the mass of the sample. Define specific heat capacity.
Heat capacity is an extensive property. Specific heat is the thermodynamic property which states the amount of heat required for a single unit of mass of a substance to be raised by one degree of temperature. Specific heat capacity synonyms specific heat capacity pronunciation specific heat capacity translation English dictionary definition of specific heat capacity.
The corresponding intensive property is the specific heat capacity. It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. The term heat capacity can be misleading since heat q is the term given to the addition or removal of energy across a barrier to a substance or system as a resul.
It is proportionality constant between the heat and temperature. Where represents the amount of heat needed to uniformly raise the temperature of the sample by a small increment.