Solve this problem by plugging the values into the Henderson-Hasselbalch equation for a weak acid and its conjugate base. Derivation of The Henderson Hasselbalch Equation.
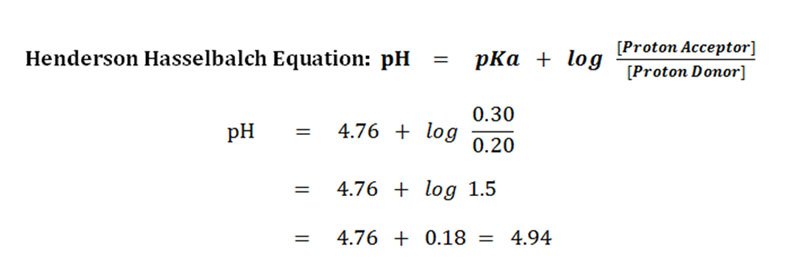 Solved Problems Henderson Hasselbalch Equation Ph Pka Easy Biology Class
Solved Problems Henderson Hasselbalch Equation Ph Pka Easy Biology Class
The Henderson-Hasselbalch equation is described and an example problem of how to calculate the pH of a buffer solution using the equation is given.
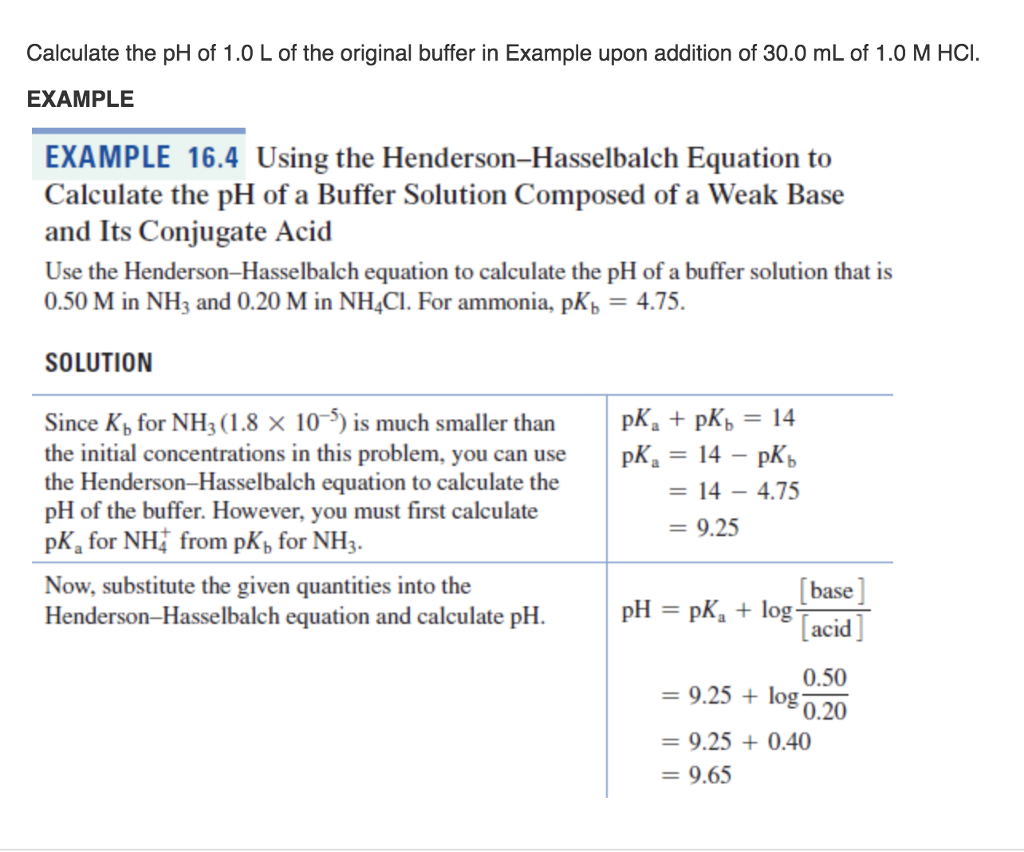
Henderson hasselbalch equation examples. The Henderson-Hasselbalch equation is written as. Thus we only need to convert the Ka into pKa. To derive the equation a number of simplifying assumptions have to be made.
Henderson Hasselbalch Equation Determine the pH of an aqueous solution of 10 mL of 003 M acetic acid and 15 mL of 0025 M acetate. It can be derived from the equilibrium constant expression for a dissociation reaction of the general weak acid HA in Equation 13. Henderson-Hasselbach equation A buffer is a solution that can resist changes in pH when small amounts of acid or base is added.
The formula for Henderson Hasselbalch is as follows. Contoh Soal Penerapan Persamaan Henderson-Hasselbalch. Note- Make sure the units for the A- and HA are in molar M units.
When it dissolved in water it is not completely ionised but achieves equilibrium so that it can exist both in ionized as well as unionised forms. The Henderson-Hasselbalch equation provides a relationship between the pH of acids in aqueous solutions and their pK a acid dissociation constant. In 1908 Lawrence Joseph Henderson wrote an equation that described the use of carbonic acid as a buffer solution.
PH pK a log A HA We already know the values for A- and HA. Henderson-Hasselbalch equation is a numerical expression which relates the pH pKa and Buffer Action of a buffer. Lets take a weak electrolyte such as HA which acts like weak acid.
Chemically a buffer is a solution of equimolar concentration of a weak acid such as acetic acid CH3COOH and its conjugate base such as acetate ion CH3COO. Note how decreasing the amount of base makes the buffer pH become more acidic compare to example 1. Hitung pH larutan buffer yang terbuat dari 020 M HC 2 H 3 O 2 dan 050 MC 2 H 3 O 2 -yang memiliki konstanta disosiasi asam untuk HC 2 H 3 O 2 18 x 10 -5.
PKa log Ka log 18 x 10-5 47. Later Karl Albert Hasselbalch re. Example Problem Applying the Henderson-Hasselbalch Equation Calculate the pH of a buffer solution made from 020 M HC 2 H 3 O 2 and 050 M C 2 H 3 O 2- that has an acid dissociation constant for HC 2 H 3 O 2 of 18 x 10 -5.
This chemistry video tutorial explains how to calculate the pH of a buffer solution using the henderson hasselbalch equation. For example the acid may be acetic acid and the salt may be sodium acetate. Then insert them into the Henderson-Hasselbalch Equation.
Example Problem Applying the Henderson-Hasselbalch Equation Calculate the pH of a buffer solution made from 020 M HC 2 H 3 O 2 and 050 M C 2 H 3 O 2- that has an acid dissociation constant for HC 2 H 3 O 2 of 18 x 10 -5. Here lets see how we can calculate it along with few examples. Solve this problem by plugging the values into the Henderson-Hasselbalch equation for a weak acid and its conjugate base.
Deriving the Henderson-Hasselbalch Equation. PHpK_alog_10fracA-HA Where pK_a is the acid dissociation constant A- is the molar concentration of the conjugate base and HA is the molar concentration of the weak acid. The pH of a buffer solution can be estimated with the help of this equation when the concentration of the acid and its conjugate base or the base and the corresponding conjugate acid are known.
In its general form the HendersonHasselbalch equation is a useful expression for buffer calculations. It is a mixture of a weak acid and its salt of a strong base an acidic buffer ORit is a mixture of a weak base and its salt of a strong acid a basic buffer. Now let us take a look at Henderson Hasselbalch equation derivation.
14 K H A HA where K is the equilibrium constant at a given temperature. Forms of the Henderson-Hasselbalch equation Finding the pH of a buffer made by mixing pyruvic acid with sodium pyruvate Calculating the ratio of the concentrations of acetate and acetic acid needed. Example Question 1.
Insert all knowns into Henderson-Hasselbalch equation and calculate the unknown pH. PKa of acetic acid is 475. You need to produce a buffer solution that has a pH of 5270.
A buffer is a solution which can resist the change in pH. Example Question 1. You can demonstrate that to yourself by calculating the new molarities in 0500 L them adding the two solutions together thereby cutting the molarities in half.
It explains the concept compo. Henderson Hasselbalch Equation NaOH is added to a 500mL of 2M acetic acid. You will get 4700 for your answer.
If the pK a value of acetic acid is approximately 48 what volume of 2M NaOH must be added so that the pH of the solution is 48. The HendersonHasselbalch equation relates the pH of a solution containing a mixture of the two components to the acid dissociation constant K a and the concentrations of the species in solution. PH pK a log base acid x 4752 log 0800 100 x 4752 0097 4655.
The Henderson-Hasselbalch Equation done in the Internet way. PH pK a log A HA.
Ethanol reacts with strong oxidizing agents such as H KMnO 4 H K 2 CrO 4 H K 2 Cr 2 O 7 to give ethanoic acid. The chemical formula for ethanol is CH 3 CH 2 OH or C 2 H 5 OH condensed structural formulas The molecular formula for ethanol is C 2 H 6 O and its molar mass is 46068 gmol.
Ethanol Formula Boiling Melting Point Ph Density Solubility
Direct link to this balanced equation.
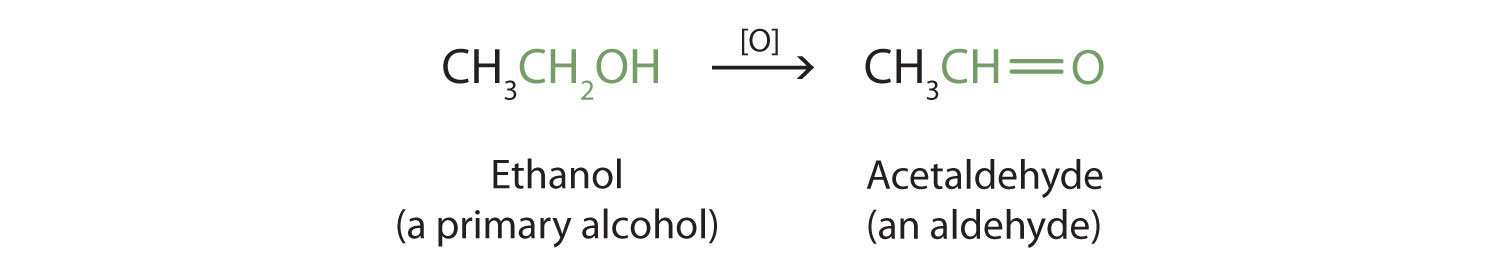
Chemical equation for ethanol. The molecular formula for ethanol is CH 3 CH 2 OH or C 2 H 5 OH. Ethanoic acid reacts with LiAlH 4 ether to give ethanol. Ethanol and ethanoic acid chemical properties.
Ethanoic acid to ethanol reaction. Ethanol is a chemical compound made up of carbon hydrogen and oxygen atoms. Ethanol is a clear colourless liquid with a smell that is referred to as the characteristic alcoholic odour.
This chemical formula can also be written as CH 3 CH 2 OH or C 2 H 5 OH. In chemistry terms ethanol is an alcohol also referred to as ethyl alcohol or drinking alcoholAs shown in figure 1 ethanol has the chemical formula Ethanol can be used as a fuel since it can produce heat energy as a result of alcohol combustion. The answer will appear below.
The chemical formula of ethanol is CH_3CH_2OH. Back in Lesson 2 I included a chemistry tutorial on some of the basic constituents of fuels. Ethanol is an alcohol compound.
Yeast is a single cell organism and a type of fungi. Ethanol also called ethyl alcohol grain alcohol or alcohol a member of a class of organic compounds that are given the general name alcohol s. A chemical reaction called fermentation takes place in which the glucose is broken down to ethanol by the action of enzymes in the yeast.
Only 5 of the ethene is converted into ethanol at each pass through the reactor. Five years later Archibald Scott Couper published the structural formula of ethanol. Pure ethanol is used as solvent for esters medicines solvent based paints and perfumes.
Ethanol is a compound of carbon hydrogen and oxygen elements was described by Antoine Lavoisier and its chemical formula was determined by Nicolas-Theodore de Saussure in 1808. It is a simple alcohol with the chemical formula C 2 H 6 O. Therefore both compounds have different chemical properties.
The molecular formula describes the type and number of atoms of elements present in an ethanol molecule. You may see ethanol written as EtOH where the Et represents the ethyl group C 2 H 5. C 2 H 6 Oethanol 3 O 2 2 CO 2 3 H 2 O Reaction type.
The chemical equation of the oxidation of ethanol with chromic acid Notice that ethanol has only one carbon-oxygen bond but in the product there is a new oxygen atom and a new carbon-oxygen. Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOH. Ethanol is a very important industrial chemical which is used as a solvent in the synthesis of other organic chemicals and as an additive to gasoline in the automotive industry forming a mixture called gasohol.
In this lesson we will be discussing the production of ethanol CH 3-CH 2-OH and butanol CH 3-CH 2-CH 2-CH 2-OH from starch and sugarEthanol or ethyl alcohol is a chemical that is volatile colorless and flammable. It is also used as a cleaning product methylated spirits. The chemical formula also may be written as CH 3 CH 2 OH.
Instructions on balancing chemical equations. Ethanol CH3CH2OH or C2H6O CID 702 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Ethanol is also the key component in almost all alcoholic drinks such as beer wine and spirits.
The reaction is reversible and the formation of the ethanol is exothermic. The equation for the reaction is. The empirical formula of ethanol is C 2 H 6 O.
Ethanols chemical formula is C 2 H 6 O. Ethanol is manufactured by reacting ethene with steam. Enter an equation of a chemical reaction and click Balance.
Ethanoic acid is carboxylic acid. Ethanol also called ethyl alcohol grain alcohol drinking alcohol spirits or simply alcohol is an organic chemical compound. Its molecular formula is C 2 H 5 OH.
The formula for ethyl alcohol or ethanol is C 2 H 5 OH or CH 3 CH 2 OH. 71 Ethanol Production - General Information. The shorthand formula is simply EtOH which describes the ethane backbone with a hydroxyl group.
By removing the ethanol from the equilibrium mixture and recycling the ethene it is possible to achieve an overall 95 conversion. It is made of nine atoms that include two carbon C atoms six hydrogen H atoms. Combustion Please tell about this free chemistry software to your friends.
Convert 20 degrees Celsius to degrees Fahrenheit. Insert the F temperature measurement in the formula and then solve to find the result.
 Fahrenheit To Celsius Converter 101 Computing
Fahrenheit To Celsius Converter 101 Computing
Celsius C Fahrenheit - 32 18 If required there are worked examples below which use this formula to show how to convert a temperature in Fahrenheit to a temperature in Celsius.

Fahrenheit to celsius equation. Celsius to Fahrenheit conversion table. If you know the temperature in Fahrenheit and want to convert it to Celsius first subtract 32 from the temperature in Fahrenheit and multiply the result by fiveninth. F - 32 18 C.
C 37 F 37 32 278 C. C 59 x F-32. 37 9 5 32 The answer is 986F.
If required there are worked examples below which use this formula to show how to convert a temperature in Celsius to a temperature in Fahrenheit. C 59 x F-32 The notation C represents the temperature in Celsius and F is the temperature in Fahrenheit. C F 32 a 12 F 12 32 1111 C.
Fahrenheit CelsiusEquations What is the formula to convert degrees Fahrenheit to degree Celsius. Moreover this formula is below. T F T C 18 32.
F the Fahrenheit Scale used in the US and They both measure the same thing temperature but use different numbers. Simply take 30 off the Fahrenheit value and then half that number. C 59 x F-32 Where C Celsius measure and F Fahrenheit measure.
The Fahrenheit and Celsius scales intersect at 40 ie 40 F 40 C. A temperature interval of 1 F is equal to an interval of 59 degrees Celsius. Fahrenheit to Celsius Exact Formula If you want a more precise calculation you can use the more exact formula.
Using this calculation we determine that 100 degrees Fahrenheit is equivalent to 3778 degrees Celsius. Check here how to convert Celsius to Fahrenheit. Fahrenheit to Celsius Conversion Formula.
First you need the formula for converting Fahrenheit F to Celsius C. Note that this value isnt perfect but it might save you having to reach for a calculator or our site. Fahrenheit to Celsius Formula.
T C T F - 32 59. T F T C 95 32. Convert degrees Fahrenheit to degrees Celsius with this simple formula.
Celsius to Fahrenheit converter. Quick and easy Fahrenheit to Celsius conversion Theres a simple rule to convert Fahrenheit to Celsius that should be good enough for general use. Convert 37C to Fahrenheit.
B 22 F 22 32 555 C. Boiling water at normal pressure measures 100 in Celsius but 212 in Fahrenheit And as water freezes it measures 0 in Celsius but 32 in Fahrenheit. Replace both temperatures with T in one of the equations above and solve for T.
How to convert Fahrenheit to Celsius The temperature T in degrees Celsius C is equal to the temperature T in degrees Fahrenheit F minus 32 times 59. Degrees Celsius F - 32 5 9 The formula to convert Fahrenheit to Celsius is F minus 32 times 5 divided by 9. Fahrenheit To Celsius Formula Use this formula to convert a temperature in Fahrenheit K to Celsius F.
50F - 32 x 5556 10C. How to convert Celsius to Fahrenheit. T F 20C 95 32 68 F.
At what temperature are Celsius and Fahrenheit temperatures equal. To convert temperatures in degrees Fahrenheit to Celsius subtract 32 and multiply by 5556 or 59. One certainly requires a formula for converting the Fahrenheit scale to the Celsius scale.
After knowing the formula one can convert the Fahrenheit scale to the Celsius scale. On the Celsius scale the freezing and boiling points of water are 100 degrees apart. The Fahrenheit to Celsius is given by the formula.
The mathematical formula behind the temperature conversion from degree Fahrenheit to Celsius in C is. Absolute zero is 27315 C or 45967 F. Take off 32 of the temperature in Fahrenheit.
Fahrenheit F Celsius x 18 32. T T 95 32. After you know the formula it is easy to convert Fahrenheit to Celsius with these three steps.
The temperature T in degrees Fahrenheit F is equal to the temperature T in degrees Celsius C times 95 plus 32. As with any math calculation and conversion its good practice to double check your results. Celsius 5 9 Fahrenheit 32 This C program to convert Fahrenheit to Celsius lets the user enter the temperature value in Fahrenheit.