The volumetric heat capacity measures the heat capacity per. The SI unit of heat capacity is joule per kelvin.
The subscript p indicates that the heat capacity and specific heat capacity apply when the heat is added or removed at constant pressure.
Define specific heat capacity. The measurement techniques of the specific heat capacity and the coefficient of thermal conductivity are shown. The heat capacity in calories per gram is called specific heat. We are giving a detailed and clear sheet on all Physics Notes that are very useful to understand the Basic Physics Concepts.
Specific heat capacity noun the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Where represents the amount of heat needed to uniformly raise the temperature of the sample by a small increment. Therefore the ratio between Cp and Cv is the specific heat ratio.
It is an intensive property as it is independent of the quantity or size of the matter. The heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Specific heat capacity is a measure of the amount of heat necessary to raise the temperature of one gram of a pure substance by one degree K.
Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The specific heat capacity of a substance usually denoted by is the heat capacity of a sample of the substance divided by the mass of the sample. The amount of heat required to raise the temperature of unit mass of the substance through 1C is called its specific heat.
C p for constant pressure Also called. The specific heat capacity of a material is a physical property. It is the ratio of two specific heat capacities Cp and Cv is given by.
The molar specific heat capacity of a substance is nothing but the amount of heat you need to provide to raise the temperature of one gram molecule of the substance through one degree centigrade. It is denoted by C. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass.
Specific heat capacity in British English. The main difference between specific heat and heat capacity is that specific heat is the amount of energy needed to raise the temperature of a given sample by 1 K while heat capacity is the amount of energy needed to raise the temperature of 1 kg of substance by 1 K. Heat capacity ratio of heat absorbed by a material to the temperature change.
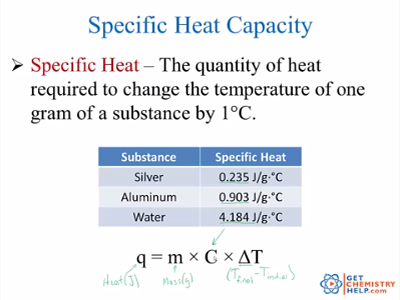
The specific heat capacity of water is 4200 Joules per kilogram per degree Celsius JkgC. Learn more about it in this article. This is because of the reason that we defined unit of heat calorie by making use of water.
It is also an example of an extensive property since its value is proportional to the size of the system being examined. Specific heat capacity derivation and definition The specific heat capacity indicates how much heat must be absorbed by a substance of mass 1 kg in order to increase its temperature by 1 K 1 C. This means that it takes 4200 J to raise the temperature of 1 kg of water by 1C.
The Heat Capacity at Constant Pressure Cp Heat capacity at Constant VolumeCv The isentropic expansion factor is another name for heat capacity ratio that is also denoted for an ideal gas by g gamma. Usually measured in joules per kelvin per kilogram. Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to a given mass of a material to produce a unit change in its temperature.
Definition Units Types Thermometry and Calorimetry. The corresponding intensive property is the specific heat capacity. Scientists needed a quantity that has no dependence on the quantity or size of matter under consideration for thermodynamic studies this made them define specific heat capacity.
Usually measured in joules per kelvin per kilogram Symbol. What is Specific Heat Capacity. Specific heat capacity synonyms specific heat capacity pronunciation specific heat capacity translation English dictionary definition of specific heat capacity.
2014 SPECIFIC HEAT CAPACITY AND THERMAL CONDUCTIVITY OF HEAT STORAGE MATERIALS BASED ON PARAFFIN BROWN-COAL WAX AND POLYETHYLENE WAX Problems of the Regional. Specific Heat Capacity Definition. Specific heat of water is taken to be 1.
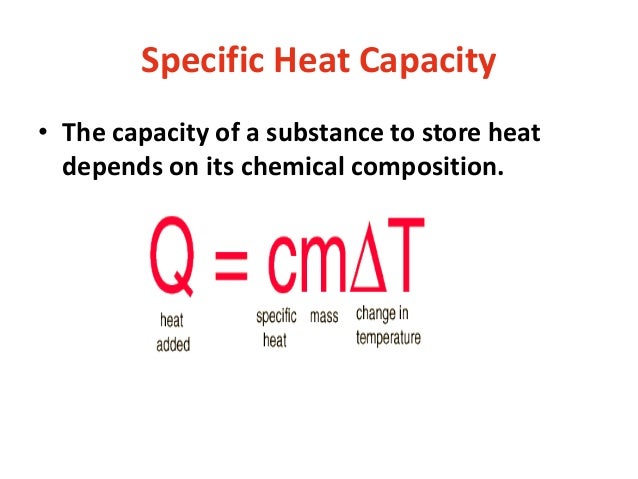
Cp Q mDT. Define specific heat capacity. What is Heat Capacity.
Like the heat capacity of an object the specific heat of a substance may vary sometimes substantially. It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. Cp for constant pressure.
Specific Heat Capacity The heat capacity of a substance per unit mass is called the specific heat capacity cp of the substance. Snezhkin Yu Mykhailyk V Korinchevska T Vorobiev L Dekusha L. Heat capacity is an extensive property.
N the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Specific heat capacity for any substance or matter can be defined as the amount of heat energy required to raise the temperature of a unit mass of that substance by one degree Celsius.
Use the formula q mDH v in which q heat energy m mass and DH v heat of vaporization. Energy is gained when the liquid turns into gas.
 Week Ending 9 25 15 Emanuel Cardenas
Week Ending 9 25 15 Emanuel Cardenas
The vaporization process requires an increase in energy to allow the liquid particles to overcome intermolecular attractions and vaporize.

Heat of vaporization examples. Similarly you have the heat of vaporization. The molecules on a surface are usually the first to undergo a phase change. M 145 grams.
So heat of fusion. Q m DH v. Water has a heat of vaporization value of 4065 kJmol.
The energy of a moving object measured in Joules. Heat of Vaporization Example Water in a teapot undergoes an increase in temperature when heat is provided by the flame of your stove. Q mDH f q 25 gx540 calg q 13500 cal Answer.
The heat of vaporization is the fundamental quantity that determines the experimental conditions at which an industrial or laboratory-scale distillation should be run. Thus the heat of fusion of ice for H 2 O18 g is 1440 cal and the heat of vaporization of water at 100A for the same quantity 9670 cal. Where Q Energy m mass DH v heat of vaporization.
What energy in. A considerable amount of heat energy 586 calories is required to accomplish this change in water. The heat of vaporization is different for all substances but is a constant for each individual substance.
Q 25 gx2257 Jg q 56425 J Part II. As a result of the network of hydrogen bonding present between water molecules a high input of energy is required to transform one gram of liquid water into water vapor an energy requirement called the heat of vaporization. Solved Examples for Heat of Vaporization Formula.
For comparison the heats of vaporization of methane ammonia and hydrogen sulfide are 816 kjmol equivalent to 12159 calg 2326 kjmol 32644 calg and 1866 kjmol 13086 calg respectively. Just two different words for the same thing depending on what direction you going. If youre asking for specific heat of vaporization then it is defined as amount of thermal energy required to change the state of a substance of unit mass from liquid to gaseous or vice versa.
Boiling water on a stove occurs when thermal energy from the heating element is transferred to the pot and in turn to the water. Ironing is also an example of vaporization. This measurement describes the amount of energy it takes to raise the temperature of water 1 degree Celsius.
An example of vaporization is when youre cooking pasta. Older units such as k cal mol calg Btu lb and others are still used sometimes. H v heat of vaporization.
26- Cloud Formation. The heat of vaporization of water is 2257 Joulesgram or 540 caloriesgram. Now rearranging the heat of vaporization equation as.
This is extremely important for life on Earth. The important thing is the number 333. Given parameters are H_v 2257 joule per gram.
The amount of heat consumed or released in a system at constant pressure. As such water also has a high heat of vaporization. In fact water takes over 40000 Joules per mole to vaporize.
When heat is added to a system from the. To do so you boil water. These two heat of vaporization example problems will show how to apply heat of vaporization to heat equations.
Vaporization or Evaporation the transition of molecules from a liquid to a gaseous state. When enough energy is supplied liquid water expands to form water vapor and the water boils. Water in its liquid form has an unusually high boiling point temperature a value close to 100C.
The amount of energy required is called the heat of vaporization. An enormous amount of energy is released when water boils. When the phase change is between liquid and gas the amount of energy per unit mass is called the heat of vaporization.
This can have example values as this remains constant at standard conditions like for Helium it is 21 kJkg for water it is 226476 kJKg for oxygen it is 213 kJkg and for nitrogen it is 20. Its called the heat of fusion because when you fuse something together you make it solid. Heat of Vaporization Example Problem 1.
The equation to find this energy is rather simple. Some irons require water which is then evaporated and allows to iron the fabric. The concentration of a gas is given by its vapor pressure.
Water has high specific heat. Lets say we heat one kilogram of room temperature water to. Heats of vaporization versus temperature.
Clouds are formed by water mixed with other chemical components such as oxygen and helium. So it could also be considered the heat of melting. The space and.
When the water boils steam rises from the water. Hot springs are vaporized waters where the heat relaxes the muscles of the body. Heat of vaporization values are usually reported in measurement units such as J mol or kJmol and referred to as the molar heat of vaporization although Jg or kJ kg are also often used.
Daily life is filled with examples of latent and sensible heat. Use Heat of Vaporization Formula. Then calculate the amount of heat energy that we need to apply to vaporize 145 grams of water.
Heat of Vaporization of Water. Q H_v times m. Uses vapor pressures at two temperatures and the Clausius-Clapeyron equation to determine the heat of vaporization.
If the heat of vaporization for water is 2257 joule per gram. Made by faculty at the University of Colo.
Specific heat is a particular type of heat capacity. Specific heat capacity definition.
 Specific Heat Capacity Spm Physics Form 4 Form 5 Revision Notes
Specific Heat Capacity Spm Physics Form 4 Form 5 Revision Notes
Like the heat capacity of an object the specific heat of a substance may vary sometimes substantially.
Specific heat capacity definition. Varying ranges of specific heat values are seen for substances depending on the extent to which they absorb heat. N the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Specific Heat Capacity Unit.
Heat capacity is an extensive property. C p for constant pressure Also called. Heat Capacity is the amount of heat required to increase the temperature of a substance to 1 degree Celsius C or 1 Kelvin whereas specific heat is the amount of heat required to increase the temperature of substance having mass 1kg or 1g by 1 degree Celsius C or 1 Kelvin.
Specific Heat Capacity Definition Specific heat capacity is the amount of heat energy required to raise the temperature of a substance per unit of mass. We are giving a detailed and clear sheet on all Physics Notes that are very useful to understand the Basic Physics Concepts. Calculating thermal energy changes.
Hence its derived SI unit is J kg1 K1. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to a given mass of a material to produce a unit change in its temperature.
A specific heat capacity calculator is functioned to deliver the outcomes along with standardized units. Meaning pronunciation translations and examples. The SI unit of heat capacity is joule per kelvin.
The heat capacity of a substance per unit mass is called the specific heat capacity cp. Specific heat capacity noun the heat required to raise unit mass of a substance by unit temperature interval under specified conditions such as constant pressure. Specific heat gives the heat capacity per kilogram of a substance.
The heat required to raise unit mass of a substance by unit temperature interval under. Specific heat capacity derivation and definition The specific heat capacity indicates how much heat must be absorbed by a substance of mass 1 kg in order to increase its temperature by 1 K 1 C. The amount of heat required to raise the temperature of unit mass of the substance through 1C is called its specific heat.
Definition of Specific heat capacity revealed that it is the amount of heat required to increase the temperature of 1 kilogram of any substance by 1 kelvin. It is also an example of an extensive property since its value is proportional to the size of the system being examined. Heat capacity C is the amount of energy needed to raise the temperature of a specific substance by 1 degree Celsius.
Usually measured in joules per kelvin per kilogram Symbol. Definition of Specific Heat and Heat Capacity Heat capacity gives the amount of energy required to raise the temperature of a given sample of a substance by 1 o C. Learn more about it in this article.
The volumetric heat capacity measures the heat capacity per. The specific heat capacity of a material is a physical property. Specific heat capacity is a measure of the energy required to raise the temperature of 1 kg of material by 1C.
The heat capacity in calories per gram is called specific heat. Heat capacity can also be viewed as the ratio of the amount of energy. The specific heat capacity of a material is the energy required to raise one kilogram kg of the material by one degree Celsius C.
Heat capacity ratio of heat absorbed by a material to the temperature change. The specific heat capacity of a substance usually denoted by is the heat capacity of a sample of the substance divided by the mass of the sample. Define specific heat capacity.
Heat capacity is an extensive property. Specific heat is the thermodynamic property which states the amount of heat required for a single unit of mass of a substance to be raised by one degree of temperature. Specific heat capacity synonyms specific heat capacity pronunciation specific heat capacity translation English dictionary definition of specific heat capacity.
The corresponding intensive property is the specific heat capacity. It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. The term heat capacity can be misleading since heat q is the term given to the addition or removal of energy across a barrier to a substance or system as a resul.
It is proportionality constant between the heat and temperature. Where represents the amount of heat needed to uniformly raise the temperature of the sample by a small increment.