Molarity is a derived unit molesLiter. One of the solutions is a standard solution of known concentration and is delivered from a burette.
 How Do We Find Unknown Concentrations Of Acids
How Do We Find Unknown Concentrations Of Acids
The resulting solution contains.

How to find concentration. To find the molar concentration of a solution use the concentration formula. Calculating the Hydronium Ion Concentration from pH. Next convert the solvent to liters.
How Does Concentration Work 5E. Instead they make concentrated stock solutions and then make dilutions of those stocks as necessary for a given experiment. How to calculate customer concentration.
If a spell has the duration of concentration youre putting a lot of your focus. Concentration changed from Dungeons and Dragons 35 in that 5E simplified and changed the mechanic. Though there are many methods by which to report the concentration molarity M is one of the most common and has units of moles per liter.
100 g of pure acid was present in the solution. Chemists like reporting concentration in Molarity because it ties well with the mole concept. Therefore you can use it as conversion factor to convert from moles to liters and vice versa.
C stands for the change in concentrations. Steps to Calculate Equilibrium Concentration. Take different known concentration solution of graphene and after measuring the.
The hydronium ion concentration can be found from the pH by the reverse of the mathematical operation employed to find the pH. TextConcentration in PPM fractextAmount of solutetextAmount of whole solution 106 You can break it down into a two-step process if that helps. Real-life chemists in real-life labs dont make every solution from scratch.
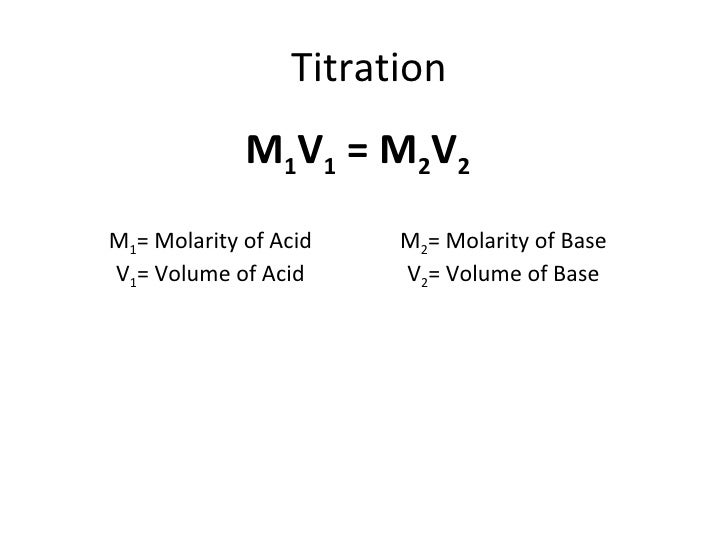
If youre converting from milliliters you may need to look up the solutes density and then multiply that by the volume to convert to grams. Concentrated phosphoric acid H 3 PO 4 was added slowly to water to produce 50 dm 3 of diluted acid solution. The technique involves determining accurately the volume of the standard solution needed to react exactly with a known volume of another.
To calculate the concentration. Titration is a very useful laboratory technique in which one solution is used to analyse another solution. I stands for initial concentration.
Mass percent composition also called mass percent or percent composition is the easiest way to express the concentration of a solution because no unit conversions are required. H 3 O 10-pH or H 3 O antilog - pH Example. Problem 3- Calculate the time it takes for the blood concentration of a drug to decrease by a given percentage.
How to Calculate Mass Percent Concentration of a Solution. Calculating the concentration of a chemical solution is a basic skill all students of chemistry must develop early in their studies. How does titration determine concentration.
What is the hydronium ion concentration in a solution that has a pH of 834. To make a dilution you simply add a small quantity of a concentrated stock solution to an amount of pure solvent. Concentration refers to the amount of solute that is dissolved in a solventWe normally think of a solute as a solid that is added to a solvent eg adding table salt to water but the solute could easily exist in another phase.
If this amount from 1 above is less than eight percent 008 you do not have a customer concentration risk. C A e epsilon x d Where C The sample concentration in mol L or g mL D Cuvette path length in cm E epsilon sample specific constant describing how much the sample absorbs at a given wavelength Qualitative Analysis. Then you can use the following formula to find the concentration.
Simply use a scale to measure the mass of the solute and the final solution and express the ratio as a percentage. 834 - log H 3 O. Calculate the concentration of the salt solution in g dm-3.
Problem 2- Calculate the value of r given data on the concentration of a drug in the blood over time. Divide the revenue from your top customer for the last twelve months or calendar year by the total gross revenue of your business for the last twelve months or calendar year. Divide the total moles of solute by the total volume of the solution in liters.
There are a few steps that need to be carried out to find the equilibrium concentration of a chemical reaction. Calculating concentration in mol dm-3. You can find the unknown concentration with the help of UV visible spectroscopy by piloting the celebration curve.
If youve ever been confused about how Concentration works then read on with our How Does Concentration Work 5E Guide. Problem 1-Find the concentration of drug in the blood at a given time after injection. To calculate the concentration of a solution start by converting the solute or the substance being dissolved into grams.
The calculation of concentration is governed by the Lambert-Beer Law. E stands for equilibrium concentration. This is the first step toward calculating the concentration of H in the solution based on the equation pH -1 log H where log is short for base 10 logarithm and the square brackets around H stand for concentration Multiplying the pH by -1 puts this equation in the form logH pH.
The steps are as below. First divide the amount of the solute by the amount of the whole solution to get a parts per 1 measurement for. What is the concentration of the acid in g dm-3.
Similarly if mass of solute is given you must convert mass to moles before you calculate solution concentration.