Elektron merupakan partikel subatomik lalu dari hal tersebut Thomson berhipotesis. Plum Pudding Atomic Theory.
 J J Thomson Atomic Model Ppt Video Online Download
J J Thomson Atomic Model Ppt Video Online Download
Thomson proposed that the shape of an atom resembles that of a sphere having a radius of the order of 10-10 m.

Jj thomson atomic theory. Thomson knew that the atom had positively and negatively. In Thomsons model the atom is composed of electrons which Thomson still called corpuscles. Through his work Thomson disproved the idea that atoms are indivisible.
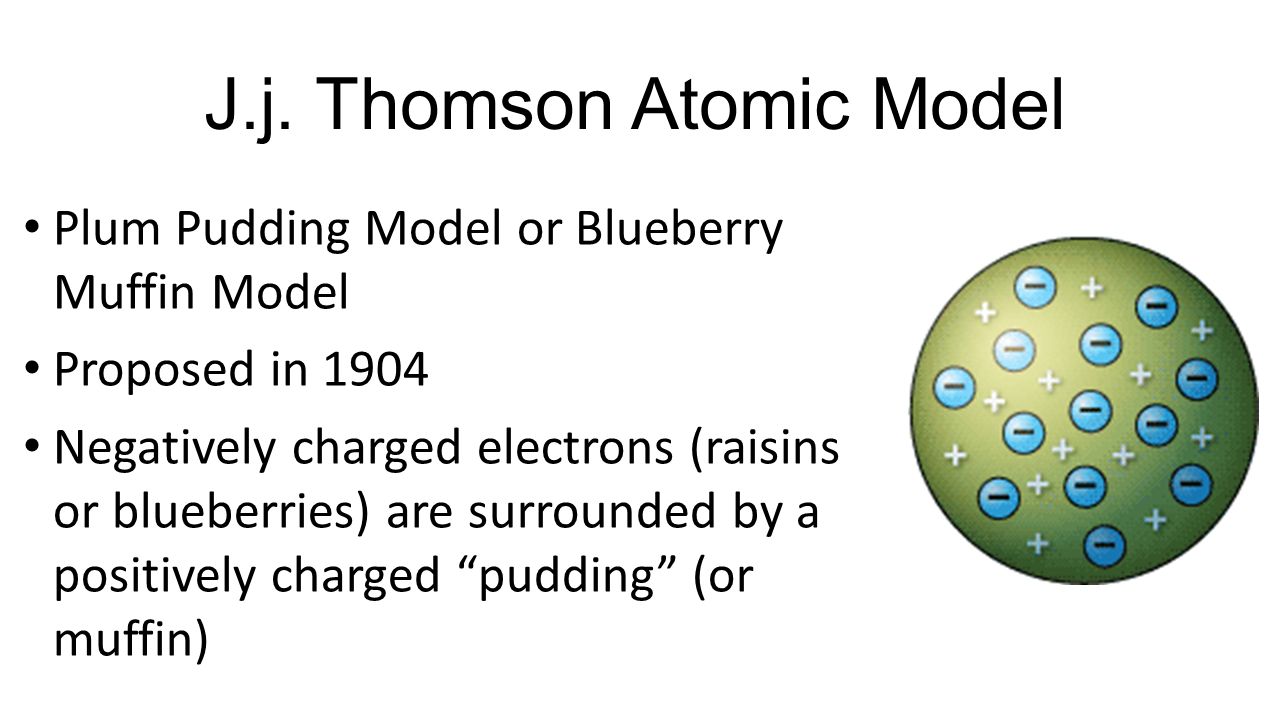
Thomson discovered the electron by experimenting with a Crookes or cathode ray tube. Thomson yang menemukan bahwa aliran tersebut ini dibelokkan ke arah plat kutub positif. Thomson who discovered the electron in 1897 proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.
Thomsons Atomic Model also called as Plum Pudding Model was the most accepted Atomic Model during the year 1904-1910 which emphasized on the inner structure of the Atom. This post will discuss what is Thomsons Atomic Model postulates of JJ. JJ Thomsons Atomic Model and Theory.
Thomson membuktikan bahwa aliran tersebut terbentuk dari partikel kecil dari atom dan juga partikel tersebut bermuatan negatiflalu kemudian JJ. Thomson discovered the first subatomic particle the electron and his new model in 1897. Instead they are made of even smaller particles.
Thomson seorang fisikawan yang berasal dari inggris yang menemukan elektron suatu partikel bermuatna negatif yang lebih ringan daripada atom di tahun 1897. Thomson 1904 On the Structure of the Atom. It was strongly supported by Sir Joseph Thomson who had discovered the electron earlier.
The atomic theory of JJ Thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Home Timeline Atomic Theory Chemists Pictures. Thomson English physicist who helped revolutionize the knowledge of atomic structure by his discovery of the electron 1897.
Thomsons experiments with cathode rays led him to discover the electron the atoms negatively charged particle. Models In 1897 JJ. Thomson 1856-1940 was the first to offer evidence from scientific experimentation showing that the atom was not the basic and indivisible unit of matter it was previously thought to be.
Thomson also contributed to the discovery of the isotope and the atoms of the same element with different atomic weights. Thomson proposed his model of the atom in 1903then only electrons and protons were known to be present in the atom. This model explained the description of an inner structure of the atom theoretically.
In addition he also studied positively charged particles in neon gas. Thomsons Model How does Plum Pudding Model Work applications and limitations. His father was a bookseller who planned for Thomson to be an engineer.
He demonstrated that cathode rays were negatively charged. Thomson lived at a time when experimentation with charged particles was uncovering a lot about the secrets of atoms. He is one of the most influential people in the atomic study.
This article will cover atomic theory by JJ Thomson his early life and other atomic theories after his discovery. An Investigation of the Stability and Periods of Oscillation of a number of Corpuscles arranged at equal intervals around the Circumference of a Circle. The British physicist JJ.
He received the Nobel Prize for Physics in 1906 and was knighted two years later. During cathode ray tube experiment a negatively charged particle was discovered by JJ. Thomson Model of an atom.
Here is a brief biography of Thomson and interesting facts about his atomic theory. Thomsons atomic atomic model was called the Plum Pudding Atomic Model and it was based on the idea that electrons are negatively charged particles scattered through out the positively charged atom. Thomson atomic model was proposed by William Thomson in the year 1900.
Pengertian Kelebihan Kelemahan Contoh Gambar Kimia Ciri-Ciri Joseph John Thomson atau JJ. Learn more about his life career and legacy. Joseph John Thomson who was always called JJ was born in Cheetham Hill England near Manchester in 1856.
He specifically discovered the existence of a negatively charged particle called the electron. Thomson was the first scientist to propose a model for structure of an atom. Thomson an English scientist proposed the famous Thomson atomic model in the year 1898 just after the discovery of electrons.
With Application of the Results to the Theory of Atomic Structure Philosophical Magazine Series 6 Volume 7 Number 39 pp. Powered by Create your own unique website with customizable templates. There are also no protons and neutrons in this model.
While Thomson was right about the existence of electrons he was wrong on where they are located within the atom. Thomson atomic model earliest theoretical description of the inner structure of atoms proposed about 1900 by William Thomson Lord Kelvin and strongly supported by Sir Joseph John Thomson who had discovered 1897 the electron a negatively charged part of every atomThough several alternative models were advanced in the 1900s by Kelvin and others Thomson held that atoms are uniform. Thomson is the scientist who discovered the electron.
Thomson menamakannya dengan elektron. Karena elektron bermuatan negatif. JJ Thomson called his atomic model the Plum Pudding model because the electrons reminded him of plums surronded by pudding positive areas.
This model showed the atom having no structure. Thomsons model showed the atom as a positively charged ball of matter with negatively chaged electrons floating freely around inside of it.
Atomic Mass Versus Mass Number The mass number is the sum of the number of protons and neutrons in an atom. Are all the Subatomic Particles considered while measuring Atomic.
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png) Difference Between Atomic Weight And Atomic Mass
Difference Between Atomic Weight And Atomic Mass
Although the SI unit of mass is kilogram the atomic mass is often expressed in the non-SI unit dalton where 1 dalton is defined as 112 of the mass of a single carbon-12 atom at rest.

What is atomic mass. A single atom has a set number of protons and neutrons so the mass is unequivocal wont change and is the sum of the number of protons and neutrons in the atom. Atomic mass is the sum of the masses of the protons neutrons and electrons in an atom or the average mass in a group of atoms. Electrons contribute so little mass that they arent counted.
Though technically incorrect the term is also often used to refer to the average atomic mass of all of the isotopes of one element. Atomic Mass is an extremely. M a is the mass of a single atom of a chemical element.
Atomic mass m a is the mass of an atom. Da or u is a unit of mass widely used in physics and chemistry. It includes the masses of the 3 subatomic particles that make up an atom.
Atomic Mass or Weight Definition Atomic mass which is also known as atomic weight is the average mass of atoms of an element calculated using the relative abundance of isotopes in a naturally occurring element. Atomic mass of an element may be defined as the average relative mass of an atom of the element as compared with mass of an atom of carbon C-12 isotope taken as 12 amu. Thus the numeric value of the atomic mass when expressed in daltons has nearly the same value as the.
The atomic mass is carried by the atomic nucleus which occupies only about 10 -12 of the total volume of the atom or less but it contains all the positive charge and at least 9995 of the total mass of the atom. Atomic mass - chemistry the mass of an atom of a chemical element expressed in atomic mass units atomic weight relative atomic mass mass - the property of a body that causes it to have weight in a gravitational field. But how is it exactly Measured.
The atomic mass constant denoted m u is defined identically giving m u m12 C12 1 Da. The mass number is a count of the total number of protons and neutrons in an atoms nucleus. However electrons have so much less mass than protons and neutrons that they dont factor into the calculation.
This second definition is actually the relative atomic. In chemistry an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon -12. It is expressed as a multiple of one-twelfth the mass of the carbon-12 atom 1992646547 10 23 gram which is assigned an atomic mass of 12 units.
It is a weighed average of the different isotopes of an element. The atomic mass is the mass of an atom. Atomic mass is the weighted average mass of an atom of an element based on the relative natural abundance of that elements isotopes.
The dalton or unified atomic mass unit symbols. Atomic weight is measured in atomic mass units amu also called daltons. So the atomic mass is the sum of the masses of protons and neutrons.
However because each atom has a very small mass this is not very helpful. Atomic mass can be expressed in grams. Atomic weight ratio of the average mass of a chemical elements atoms to some standard.
This unit is commonly used in physics and chemistry to. Atomic mass is the sum of all the protons neutrons and electrons in a single atom or molecule. Atomic Mass is an extremely important concept in Chemistry.
It is a unit of mass used to express atomic masses and molecular masses. It has been found experimentally that the mass of one carbon-12 atom is 199265 10 26 kg. The mass of an isotope of an element measured in units formerly based on the mass of one hydrogen atom taken as a unit or on 116 00625 the mass of one oxygen atom but after 1961 based on 112 00833 the mass of the carbon-12 atom.
The protons and neutrons of the nucleus account for nearly all of the total mass of atoms with the electrons and nuclear binding energy making minor contributions. However the mass of an electron is so small it is considered negligible and not included in the calculation. An atomic mass symbol.
Protons neutrons and electrons. It is defined as 112 of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. Atomic mass the quantity of matter contained in an atom of an element.
In this scale 1 atomic mass unit amu corresponds to 1660539040 10 24 gram. Since 1961 the standard unit of atomic mass has been one-twelfth the mass of an atom of the isotope carbon-12. Therefore 1 u is defined as of the mass of a carbon-12 atom.
Atomic mass indicates the size of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element.
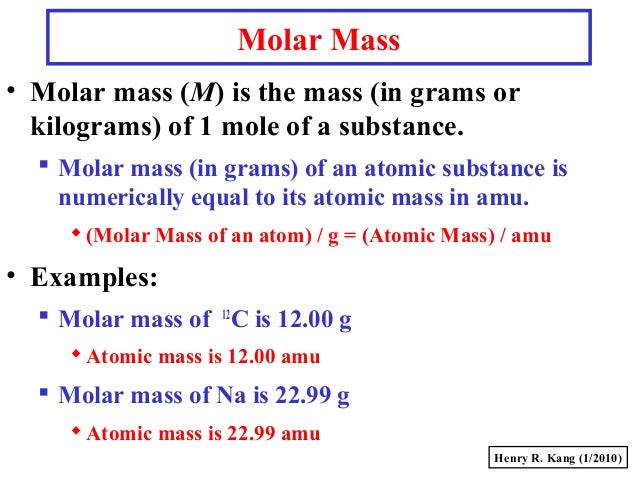
It is the mass of a mole of a substance. What is Atomic Mass.
 How To Calculate Atomic Mass Atoms And Molecules For Kids Teaching Chemistry Atom
How To Calculate Atomic Mass Atoms And Molecules For Kids Teaching Chemistry Atom
The atomic mass of an element is frequently used by chemists to determine the amount of substance required in a chemical reaction.
Atomic mass definition chemistry. The mass number reports the mass of the atoms nucleus in atomic mass units amu. It relates the mass of an element to the number of atoms. The relative atomic mass of an element is a weighted mean mass of the isotopes of an element compared with that of the 12C isotope which has a mass of exactly 12.
The daltonor unified atomic mass unitsymbols. It is defined as 112 of the mass of an unboundneutral atom of carbon-12in its nuclear and electronic ground stateand at rest. Definition of Atomic Mass Atoms are the basic units of matter.
The atomic mass is the sum of the mass of protons neutrons and electrons. Atomic Mass Unit AMU The periodic table of elements contains every atom known to mankind. Daor u is a unitof masswidely used in physics and chemistry.
The mass of an atom or a molecule is referred to as the atomic mass. Each unique atom has a unique atomic number and atomic mass. One atomic mass unit is equal to one-twelfth of the mass of an atom of carbon 12 isotope.
The number of protons in an atom of an element is called the atomic number Z. The atomic mass is used to find the average mass of elements and molecules and to solve stoichiometry problems. Medical Definition of atomic mass unit.
The mass number A of an element is the total number of protons and neutrons in an atom of that element. The symbol used for it is M. It is denoted by ma.
Atomic mass - chemistry the mass of an atom of a chemical element expressed in atomic mass units atomic weight relative atomic mass mass - the property of a body that causes it to have weight in a gravitational field. The sum of the mass number and the atomic number for an atom A-Z corresponds to the total number of subatomic particles present in the atom. Updated June 30 2019 In chemistry an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon -12.
Atomic mass definition the mass of an isotope of an element measured in units formerly based on the mass of one hydrogen atom taken as a unit or on 116 00625 the mass of one oxygen atom but after 1961 based on 112 00833 the mass of the carbon-12 atom. Relative atomic masses do not. Mass is a basic physical property of matter.
It has a unit of the unified mass unit u or the atomic mass unit amu. Atomic mass indicates the size of an atom. G mol 1 is the standard unit for the molar mass.
The atomic mass constant denoted muis defined identically giving mu m12C12 1 Da. The atomic number is the number of protons. It is a unit of mass used to express atomic masses and molecular masses.
Atomic mass is a fundamental concept in chemistry. They are the smallest components of a chemical element which is a substance that cannot be broken down into simpler material. Atomic Mass or Weight Definition Atomic mass which is also known as atomic weight is the average mass of atoms of an element calculated using the relative abundance of isotopes in a naturally occurring element.
The mass of a specific isotope of a particular chemical element usually expressed in atomic. Atomic mass of an element is the number of times an atom of that element is heavier than an atom of carbon taken as 12. The number of protons in an atom of an element is its atomic number.
A unit of mass for expressing masses of atoms molecules or nuclear particles equal to ¹₁₂ the mass of a single atom of the most abundant carbon isotope 12C called also dalton.
Atomic number is represented by Z while atomic mass by M. Much of the mass in an atom is located in the nucleus of the atom and is in the form of protons and neutrons.
 The Difference Between Atomic Numbers And Atomic Mass Youtube
The Difference Between Atomic Numbers And Atomic Mass Youtube
What is the difference between atomic number and mass number.

Difference between atomic mass and mass number. What is the difference between atomic mass and atomic number. It is represented as Da and is the standard unit used to indicate the mass of an atom. The total number of neutrons and protons of an atom is called atomic mass.
Atomic number is the number of protons in a nucleus of an atom. Atomic mass m a is the mass of an atom. The atomic number is the value found associated with an element on the periodic table because it is the key to the elements identity.
The atomic mass unit is Dalton. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Each of these particles weighs approximately one atomic mass unit.
Difference between Atomic Mass and Atomic Weight Key Difference. While mass number on the other hand is the number of protons in the nucleus of that certain atom. Atomic mass is defined as the total mass of protons neutrons and electrons present in an atom of an element.
Be careful you dont confuse atomic number and mass number. While the mass number is the sum of the protons and neutrons in an atom the atomic number is only the number of protons. While atomic mass is the sum of the protons and neutrons present in the nucleus.
Difference Between Atomic Mass and Atomic Number. Definition of Atomic Mass Atomic mass is defined as the number of protons and neutrons contained in an individual unit of a substance. Atomic mass is the mass of an atom.
What is Mass Number. Conventionally atomic number is written in the left bottom corner of an element whereas the mass number is written in the left upper corner. Electrons contribute so little mass that they arent counted.
Atomic mass is the mass of an atom and is given in amu. It is the average of all isotopes in an atom. A single atom has a set number of protons and neutrons so the mass is unequivocal wont change and is the sum of the number of protons and neutrons in the atom.
Atomic mass also atomic weight is the total weight of an element. One mole is equal to Avogadros number N A 6022 1023 atoms or molecules. Therefore it is like the combined weight of both protons and neutrons in an atom.
The main difference between atomic number and mass number is that the atomic number indicates the number of protons present in an atom whereas the mass number indicates the sum of the number of protons and the number neutrons present in an atom. What is the difference between Mass Number and Atomic Mass. The difference is that one is a count of the protons in an elements nucleus while the other is a count of both the protons and neutrons.
What is the difference between an atoms atomic number and its mass numberAtomic number Z is the of protons in an atom while the mass number A is the total of protons and neutrons in an atomAs I understand it and ask prof when you can an isotope is any variation in the number of neutrons in an atom. Atomic mass is associated with the number of neutrons and protons that are present in a particular nucleus of an element. The main difference between Atomic Mass and Atomic Number is that Atomic Mass is the total number of neutrons and protons in the nucleus of an atom whereas Atomic Number is the total number of protons existing in the nucleus of an atom.
It is calculated to 112th of the mass of carbon atom. Atomic number is usually the number of protons present in an elements nucleus. It is based on the abundance of a certain atoms isotope.
Mass number means the total number of neutrons and protons nucleons in a nucleus of an atom. Atomic number tells about the number of proton present in the atom which is equal to the number of electron present in the neural or parent atom. Atomic Mass is actually the mass of the entire particle.
Mass number is the total number of protons and neutrons in the nucleus. It is the average weight of an element. Atomic weight is defined as the ratio of average mass of the atom present in an element.
The mass number is the count of these particles and so the mass number is very close to the atomic mass. One difference between atomic mass and molar mass to take note of is that the latter refers to the weight of a mole of a substance while the latter refers to the weight of the molecules of a substance. Electrons are not counted and can be neglected as their size is very small.
Atomic mass vs Atomic number. However a molar mass is the mass of one mole atoms or molecules and is given in grams. Mass number is an integer value whereas the atomic mass often is a decimal value.
Find out in this video from the Pr. Difference Between Atomic Weight and Mass Number.
 Atomic Number Vs Atomic Mass Hydrogen Z 1 To Ununoctium Z 118 7 Download Scientific Diagram
Atomic Number Vs Atomic Mass Hydrogen Z 1 To Ununoctium Z 118 7 Download Scientific Diagram
Atomic number is used to define the type of element a material or substance is.

Atomic number vs atomic mass. Electrons do not usually weigh much so the atomic mass is calculated by adding the number of protons to neutrons. Atomic number is the number of protons in a nucleus of an atom. Mass number is the number of.
The atomic mass on the other hand is the number of both the protons and neutrons present in the nucleus of the element. It is the average weight of an element. While the mass number is the sum of the protons and neutrons in an atom the atomic number is only the number of protons.
We can characterize atoms by their atomic numbers and mass numbers. In fact the number of protons dictates what atom we are looking at eg all atoms with 6 protons are carbon atoms. For example a lithium atom Z3 A7 amu contains three protons found from Z three electrons as the number of protons is equal to the number of electrons in an atom and four neutrons 7 3 4.
Be careful you dont confuse atomic number and mass number. Key Differences between Atomic Mass and Atomic Number. Atomic Number The total number of neutrons and protons that exist in the nucleus of an atom combine to make the mass of the atom known as its atomic mass.
Atomic Number Atomic Mass and Relative Atomic Mass Atoms of each element consist of a particular number of protons. So atomic mass is the average weight of an atom. They differ in the way they are defined.
Electrons contribute so little mass that they arent counted. It has 17 positive charges and 17 negative charges meaning that it is neutral overall. Atomic number is represented by Z whereas Atomic mass is represented by A.
Every chlorine atom has 17 protons and 17 electrons. Atomic mass does not define the type of element whereas Atomic number defines the type of element. For example any atom with an atomic number of 8 its nucleus contains 8 protons is an oxygen atom and any atom with a different number of protons would be a.
Atomic number is usually the number of protons present in an elements nucleus. In a typical sample of carbon-containing material 9889 of the carbon atoms also contain 6 neutrons so each has a mass number of 12. Conventionally atomic number is written in the left bottom corner of an element whereas the mass number is written in the left upper corner.
For example the atomic number of chlorine is 17. The key difference between atomic weight and mass number is that atomic weight is the average mass calculated considering all the isotopes whereas mass number is the mass of a specific isotope. The main difference between atomic number and atomic weight is that atomic number gives the number of protons present in the nucleus whereas atomic weight gives the mass of an atom.
Atomic Number Mass Number Properties of Matter Chemistry FuseSchoolHow do we tell elements apart from each other. The atomic number represented by the letter Z of an element is the number of protons in the nucleus of each atom of that elementAn atom can be classified as a particular element based solely on its atomic number. While the mass number is the sum of the protons and neutrons in an atom the atomic number is only the number of protons.
A single atom has a set number of protons and neutrons so the mass is unequivocal wont change and is the sum of the number of protons and neutrons in the atom. Atomic mass is associated with the number of neutrons and protons that are present in a particular nucleus of an element. Atomic mass is used to show different isotopes of the same element whereas its not the case for atomic number.
Given an atomic number Z and mass number A you can find the number of protons neutrons and electrons in a neutral atom. Start studying Atomic Number vs. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes.
Element showing its atomic number and mass number. Learn vocabulary terms and more with flashcards games and other study tools. Atomic mass m a is the mass of an atom.
On the flip side the total protons numbers that are present in the atoms nucleus known as the atomic number. Mass NumberA Number of Protons Number of Neutrons The element carbon C has an atomic number of 6 which means that all neutral carbon atoms contain 6 protons and 6 electrons. Difference Between Atomic Mass and Atomic Number.
Atomic number and atomic weight are two chemical terms that are used to explain the number of subatomic particles present in an atom and its mass. Atomic number is denoted by Z and mass number is denoted by the symbol A. Mass number is the total number of protons and neutrons in the nucleus.
It is the number of protons present in an elements nucleus. Atomic number is the number of protons there in an atom.