An electrolyte is a substance that produces an electrically conducting solution when dissolved in a polar solvent such as waterThe dissolved electrolyte separates into cations and anions which disperse uniformly through the solventElectrically such a solution is neutral. NaCl - sodium chloride.
 Aqueous Solutions Of Electrolytes Online Presentation
Aqueous Solutions Of Electrolytes Online Presentation
Electrolytes are important body constituents because they Conduct electricity essential for muscle and nerve function.
What makes a strong electrolyte. In nutrition the term refers to essential minerals found in your blood sweat and urine. They completely dissociate to produce ions in a solution. Exert osmotic pressure keeping body fluids in their own compartments.
A strong electrolyte will completely dissociate into its component ions in solution. However it does not mean the chemical completely dissolves in water. A lecture presentation draft to accompany the Conductivity of Strong Electrolytes Weak Electrolyte and Non-Electrolytes Demonstration and Class Activity.
An electrolyte is composed of cations or positively charged ions and anions or negatively charged ions. The more ions in the solution the stronger the electrical conduction. An essential electrolyte for humans sodium is responsible for controlling the total amount of water in the body.
The electrolyte of a battery consists of soluble salts acids or other bases in liquid gelled and dry formats. Therefore a strong electrolyte is a solute that completely dissolves in water. Salts much have high solubility in the solvent to act as strong electrolytes.
A weak electrolyte is a poor conductor of electricity. A concentrated solution of this strong electrolyte has a lower vapor. It is also important for regulating blood volume and maintaining muscle and nerve function.
Ions are electrically charged atoms that have lost or gained electrons. An example of a weak electrolyte is acetic acid which is also a weak acid. An ionic compound for example sodium chloride dissolved in water is called an electrolyte because it conducts electricity.
Strong and Weak Electrolytes. The main advantages are the increased safety no issues of leakages of toxic organic liquids low. When these minerals dissolve in a fluid they form electrolytes positive or negative ions used in.
People throw around the term electrolyte quite a bit but what does it mean. With a greater understanding of the properties of ions in solution its definition was replaced by the present one. HCl - hydrochloric acid.
What makes something a strong electrolyte a weak electrolyte or a nonelectro. For example some species are only slightly soluble in water yet are strong electrolytes. Electrolytic cells use this principle.
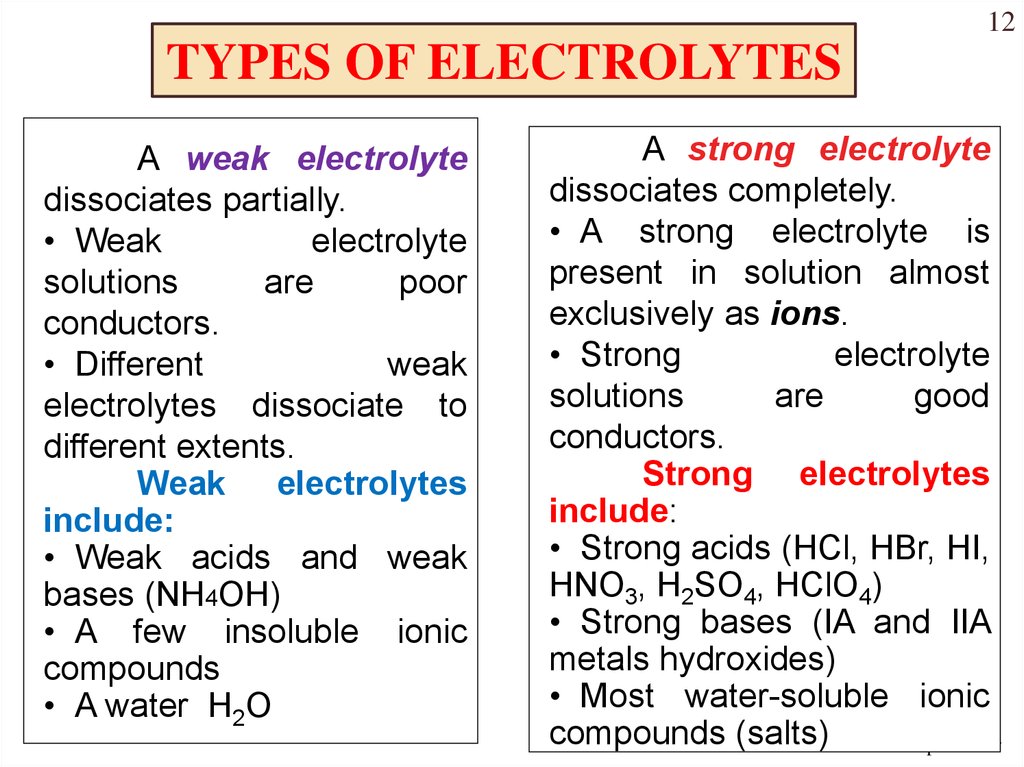
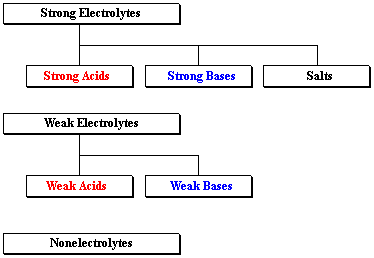
Strong acids strong bases and ionic salts that are not weak acids or bases are strong electrolytes. A strong electrolyte is a good conductor of electricity. This means 100 of the dissolved chemical breaks into cations and anions.
NaOH - sodium hydroxide. Solution solvent solute. HBr - hydrobromic acid.
A solid-state electrolyte SSE is a solid ionic conductor electrolyte and it is the characteristic component of the solid-state batteryIt is useful for applications in electrical energy storage EES in substitution of the liquid electrolytes found in particular in lithium-ion battery. Strong electrolytes readily produce ions when they are soluble. A strong electrolyte is a solutionsolute that completely or almost completely ionizes or dissociates in a solution.
Strong electrolytes include the strong acids strong bases and salts. These ions can conduct an electric current in a solution. A strong electrolyte is a compound that can completely dissociate into its ions when dissolved in water.
This presentation is available to download from the menu. Originally a strong electrolyte was defined as a chemical that when in aqueous solution is a good conductor of electricity. HCl hydrochloric acid H 2 SO 4 sulfuric acid NaOH sodium hydroxide and KOH potassium hydroxide are all strong electrolytes.
These chemicals completely dissociate into ions in aqueous solution. Includes visual images main student activities computer animations computer simulations videos and a sample of clicker questions. Strong electrolytes completely ionize in water.
These ions are good conductors of electric current in the solution. The formation of these positively and negatively charged ions called cations and anions is what allows a strong electrolyte to conduct electricity. Electrolytes - A substance that when dissolved in water produces a solution that can conduct an electric current.
HI - hydroiodic acid. Electrolyte serves as catalyst to make a battery conductive by promoting the movement of ions from the cathode to the anode on charge and in reverse on discharge. The strength of an electrolyte whether it is a strong electrolyte or a weak electrolyte depends on the substances ability to form ions by dissociation or ionization.
Sodium is the major positively-charged ion cation outside your body cells and is mostly found in blood plasma and lymph fluid. If an electric potential is applied to such a solution the cations of the solution are drawn to the electrode that has. Thus they are good electricity conductors.
A weak electrolyte on the other hand will remain mostly undissociated in solution. Strong Electrolytes conduct current very efficiently. SrOH 2 - strontium hydroxide.
Molten sodium chloride or aqueous NaCl solutions have completely dissociated into Na and Cl ions. For example ionic compounds are strong electrolytes. Aqueous aq water solution.
Home to at least 300000 residents a distinct identity that. Cities usually get this because they have a special number of people or are important.
 Is Brighton A City What Makes A Town A City Jolly Explorer
Is Brighton A City What Makes A Town A City Jolly Explorer
In the past cities got that name if they had a cathedral or a university.

What makes a town a city. Unlike the towns cities are generally well planned and have proper sanitation drinking water roads and other modern amenities. In fact a town can now apply for city status by submitting an application to the Lord Chancellor who makes recommendations to the sovereign. Generally the difference between towns and villages or hamlets is the sort of economy they have.
People in towns usually get money from industry commerce and public service not agriculture. Towns also have smaller geographical areas. City is generally a bigger residential place than a town but this is not the conclusive factor in a place being called a city.
What is a Town A town is described as an urban area that is smaller than a city but larger than a village. Competitions for new grants of city status have been held to mark special events such as coronations royal jubilees or the Millennium. Generally a mayor is the head of a city corporation while a chairman is the head of a municipality.
In some places it is a kind of local government. They tend to be less densely populated than cities and have less diversity in terms of peoples ethnicities. A city can provide local government services to its citizens.
A town must be granted city status by the British monarch. City derives from a French word meaning citizenry. In earlier times a city was a place having a cathedral in Europe.
Obviously economics play a massive part in a citys well-being given that growth is created in large part by urbanization and the desire for new jobs restaurants musicians etc. However the definition of town and city varies considerably in different countries. In Ireland the term city has somewhat differing meanings in Northern Ireland and the Republic of Ireland.
Historically city status in the United Kingdom and before that in the Kingdom of Ireland was a ceremonial designationIt carried more prestige than the alternative municipal titles borough town and township but gave no extra legal powers. But different economic circumstances and preferences Texans tend to not like taxes as much as folks in the north for example will beget different outcomes. As with cities there is more than one way to say what a town is in different countries.
In the United Kingdom a city is a town which people have always called a city or which has got the name city status by royal charter a special paper from the king or queen. The main difference between town and city is that a city is larger than a town. Traditionally in England and Wales city status was given to settlements with diocesan cathedrals though this is no longer a requirement.
Which is to say cities should embrace their unique characters of place and avoid sameness. Because of the population that provides labor and purchasing power businesses prefer to set base in them with the added advantage of government facilities. As with the words village and town city can also refer to the its inhabitants.
It may even be a city. City status is granted by the reigning monarch usually to commemorate special occasions. It officially became a city in 1925.
Pretty much every settlement no matter how extraordinary or ordinary it is can be classified as a city a town or a village. County governments typically provide services to these unincorporated communities. Town definition is - a compactly settled area usually larger than a village but smaller than a city.
Good city leaders also think about regional growth because as a metropolis expands they will need the cooperation of surrounding municipalities and regional service providers. It is granted by personal Command of The Queen on the advice of Her Ministers. In Great Britain a borough with a bishops seat is called a city.
A city will typically be larger than a town and have multiple places of worship and several meeting points. The centre of power mainly rests in the cities and not in the towns. Smart growth identifies and nurtures the very best opportunities for growth plans ways to cope with its demands integrates environmental thinking and ensures that all citizens enjoy a citys prosperity.
City status is a rare mark of distinction granted by the Sovereign and conferred by Letters Patent. When they say town people are normally thinking of a big important place. A city is a large or important town.
In the United States cities are incorporated municipalities with local governments. But what makes these places different. It was deemed to be a significantly important town for its role as the centre of the pottery industry in the area.
In UK a city is a place with a Royal Charter. An example of an English town that has been awarded city status following the new criteria is Stoke-on-Trent which does not have a cathedral. A town is usually a place with a lot of houses but not a city.
How to use town in a sentence. Finally the sixth key to making a city attractive is to make it local. Today the UKs official criteria for what constitutes a city remain opaque but those put in place in 1907 remain a good rule of thumb.
In many places in the US a town village community or neighborhood is simply an unincorporated community with no governmental powers. Towns on the other hand may share some geographic and demographic similarities with cities but in smaller sizes. Cities have bigger economies compared to towns.
According to the part of the British government known as the Department for Constitutional Affairs. Cities are generally places that have better facilities of sanitation housing and transportation.