Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water at pressures ranging from 147 to 3200 psia 1 to 220 bara. For instance almost everyone knows that the freezing point of water is 0 degrees Celsius and the boiling point of water is 100 degrees Celsius.
Pure water - microscopic view.
Boiling point of pure water. Thus we dont use the degree symbol when reading temperatures in this system. It varies from 72C to 101C accordingly from the highest point to the lowest point on land. The IUPAC recommended standard boiling point of water at a standard pressure of 100 kPa 1 bar is 9961 C 2113 F.
The Boiling Point of Pure Water at 1 ATM is 212oF or 100oC Why pure water is less time to boil than water with salt. There are two conventions regarding the standard boiling point of water. The boiling point of water varies at various locations.
The output temperature is given as C F K and R. 10 M NaCl solution - microscopic view. Choose the actual unit of pressure.
Example 1383 In Example 1381 we calculated that the vapor pressure of a 302 aqueous solution of ethylene glycol at 100C is 851 mmHg less than the vapor pressure of pure water. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure sea level. Pressure must be within the ranges 0-1000 mbara 0-147 psia 0-760 mm Hg or 0-30 in Hg.
The calculator below can be used to calculate the water boiling point at given absolute pressures. Normal boiling point 1000 o C. We dont insert the degree symbol o or the word degree to refer to a specific temperature on the Kelvin scale.
Temperature given as C F K and R. The water vapour will also condense. In this regard the boiling point of water changes with a change in barometric pressure.
Boiling water is characterized by energetic bubbles and steam and it is considered to be hot. The boiling point of a liquid varies according to the applied pressure. The boiling point of water on the Kelvin scale is 3732 K.
However there is no danger of boiling the NaCl. For pure water the boiling point is 100 degrees Celsius 212 Fahrenheit at one atmosphere of pressure and the melting point is 0 degrees Celsius 32 degrees Fahrenheit at one atmosphere of pressure. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33.
At sea level water boils at 100 C 212 F. If you boiled all the water off the ions would recombine to form solid salt. Pure water will melt at 0C and boil at 100C.
Boiling Point of NaCl. At at high altitudes the lower pressure makes the boiling point several degrees lower. Conventionally the temperature at which water boils is 100 degrees Celsius or 212 Fahrenheit but only at sea level.
The normal boiling point is 9997 C 2119 F at a pressure of 1 atm ie 101325 kPa. When you dissolve salt in water it breaks into sodium and chloride ions. Boiling and freezing points of pure substances are well-known and easily looked up.
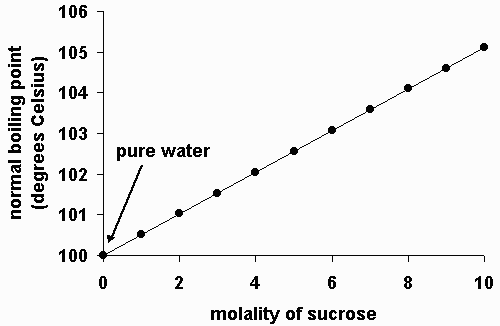
Whenever a solute such as salt is added to a solvent such as water the boiling point becomes higher than that of the pure solvent. The normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure 760 mm 2992 inches of mercury. However the value is not a constant.
The boiling point of water depends on the atmospheric pressure which changes according to elevation. Adding salt to water increases the boiling point of water due to a fundamental colligative property of matter known as boiling point elevation. Hence a 100 m NaCl solution will have a boiling point of about 10102C.
Testing its boiling point. For example the boiling point of pure water at 10 atm is 100 o C while the boiling point of a 2 saltwater solution is about 102 o C. The boiling point of pure water is lower than the boiling point of a.
The freezing point depression is the amount the freezing temperature decreases. It is important to recognize just how much the temperature of boiling water is reduced as the altitude increases. A lower boiling point means that food cooks at a lower temperature despite the fact that the water is boiling.
Every degree unit on the Celsius scale is equal to one degree unit on the Kelvin scale. Salt like other ionic solids has an extremely high boiling point. Pure substances have precise melting and boiling points.
Normal boiling point 1010 o C. The reason for these variations is the lowering of atmospheric pressure as we travel to the highest point such as mountains from lowest land point ie Dead sea. The boiling point of sodium chloride is 2575 F or 1413 C.
Therefore the boiling point elevation would be 2 o C. Note that the ionic solid NaCl produces Na ions blue and Cl-ions green when dissolved in water.
In a sample of impure salol salol mixed with other substances. In other words it is homogeneous no matter when you sample it.
An element is a substance that consists of only one type or kind of atom.
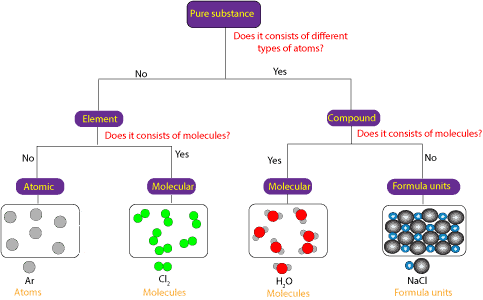
What's a pure substance. An element is an example of a pure substance. Pure substances are made of only one type of atom or molecule. In science a pure substance contains only one element or compound.
A pure substance refers to an element or a compound that has no component of another compound or element. These are the ingredients that you see listed on the. Mixtures are categorised as homogeneous and heterogeneous.
Two pure substances mixed together is known as a mixture. In chemistry a pure substance has a definite composition. A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together The components of a mixture can usually be.
Diamond is a pure substance because it is directly made up of carbon atoms. Pure substances are further classified as elements and compounds. Hydrogen consists of hydrogen atoms only while iron consists of only iron atoms.
So in addition to elements compounds and alloys a pure substance might include honey even though it consists of many different types of molecules. A pure substance or chemical substance is a material that has a constant composition is homogeneous and has consistent properties throughout the sample. Individual properties of components are retained.
Called salol C 13 H 10 O 3 and an impure sample. Mixtures can be classified into two types viz. Mineral water is mostly water but there are other substances mixed with it.
It can be a compound or a single element. A pure substance is a chemical that has definite and constant composition. Another way of saying it is that a pure substance has the same type of chemical and this chemical has the same composition throughout the substance.
It is also made up of the same particles. Pure substances are further divided into elements and compounds. Simply a pure substance is a substance which is composed by molecules or directly by atoms of only one kind.
In chemistry a pure substance consists of only one type of atom molecule or compound. Substances which have a specific composition and cannot be separated into any constituents are called pure substances. Pure substances are substances that are made up of only one kind of particles and has a fixed or constant structure.
In a sample of pure salol the temperature stays the same as it changes state. Heterogeneous and homogeneous mixtures. Examples include pure water H 2 gas gold.
What is Pure Substance. It has the same properties and the same ratioof hydrogento oxygenwhether it is isolated from a river or made in a laboratory. Hydrogen gas and pure iron are examples of pure substances.
Scientists often use filtration to separate pure substances from a mixture in order to analyze. Pure substances can be categorised as gas liquid and solid. Generally it does not matter which definition you use but if you are asked to give examples of pure substances as a homework assignment use those that meet the narrow definition such as gold silver water and salt.
Also a pure substance can be defined as any single type of material that has not been contaminated by another substance. A pure substance participates in a chemical reaction to form predictable products. Definite composition means that the makeup of the chemical is fixed and constant throughout the pure substance.
In other words it is free of contaminants. Salt water is not because it contains sodium chloride and water. Pure substances are defined as substances that are made of only one type of atom or only one type of molecule a group of.
Chemical and physical properties are constant. It participates predictably in a chemical reaction. Common Definition of a Pure Substance.
Some people define a pure substance to be a material that consists of one type of building block of matter. The combination of two or more pure substances is called a mixture. In chemistry a pure substance has a definite composition.
A common example of a chemical substance is pure water. It can be a compound or a single element. In chemistry a pure substance is a material with a constant composition.
Chemical substances are often called pure to set them apart from mixtures. By this definition only elements and compounds are pure substances while homogenous mixtures are not. A pure substance consists entirely of one type of atom or compound.
The material is no longer a pure substance if it has been mixed with another pure substance. Whats a Pure substance. A pure substance cannot be separated into any other kinds of matter by any physical or chemical process.
Chemical and physical properties may vary. If you add corn syrup to the honey you no longer have pure honey. To a non-chemist a pure substance is anything composed of a single type of material.
A pure substance is any single type of material that are made of only one type of atom or only one type of molecule. The pure substance within chemistry is a very simple concept to grasp.
Oxyfuel combustion uses pure oxygen instead of air to burn carbonaceous materials resulting in a CO 2 separation efficiency theoretically close to 100 should the fuel and oxygen be free of contaminants. Heres where it gets complicated.
 Chemistry 101 Oxygen Is Not Flammable
Chemistry 101 Oxygen Is Not Flammable
No the problem with oxygen is that while not being flammable it is much more efficient in supporting burning.

Is pure oxygen flammable. Oxygen is not flammable as it does not burn it supports burning. Pure oxygen is very very explosive. Here is a video response to the ridiculous comments about oxygen and how dangerously flammable it is.
It takes time for the hydrogen in the pure hydrogen balloon to mix with the oxygen in the air and that times lets us see a brief beautiful spread of fire. Any stoichiometric mixture of methane and oxygen will lie on the straight line between pure nitrogen and zero percent methane and 33 percent methane and 67 percent oxygen - this is shown as the red stoichiometric line. Without the fuel though no combustion will take place no matter how high the concentration of oxygen is.
It speeds up combustion. Oxygen can even make some materials burn that are not normally flammable. Is pure oxygen highly flammable.
The fire will be increased vigorously when oxygen is added to it. The only reason the atmosphere doesnt burn up when someone lights a match is because the atmosphere is also 71 nitrogen. If that is the case 21 percent of atmosphere that is made up of oxygen might have got burnt by now.
The upper and lower flammability limits of methane in oxygen are located on the methane axis as shown. Even though the oxygen by itself will not burn and even though a flame may be relatively small the oxygen will help the flame grow much larger and you could easily ignite a lethal conflagration. NAA engineers knew that in a pure oxygen environment a single spark could turn into a raging fire with explosive consequences and they made their concerns known to NASA.
For something to burn the reaction requires a fuel the thing that burns and an oxidizer like oxygen. Contrary to popular belief oxygen itself is not flammable. A fire is created when certain substances combine with oxygen typically accompanied by intense heat or a spark.
That is why smoking was stopped in rooms using oxygen. Httpsyoutuben_t7CIf0SvsSometimes in science you think you know exactly what youre talking about oth. A study published in 2017 notes that while HBOT has shown promise for wound healing and other uses there.
For burning to happen a strong oxidizer for example oxygen and a strong reducer for example carbon must be present. This chapter examines several oxyfuel systems considering two categories of power cycle those based on steam cycles and those based on gas cycles both of which generate oxygen using a cryogenic air separation unit. How do you think about the answers.
It is not flammable but it is an oxidizer. Oxygen is also not flammable but it is a high-energy gas that very readily oxidizes other materials. Applying oxygen to a burning fuel will enhance the burning process and increases the size of the flame.
Patients on oxygen therapy who are smokers are not going to burst into flame or explode if they smoke. So the short answer is pure oxygen is generally bad and sometimes toxic. Oxygen feeds fire so its dangerous to use around something that is burning because it will help the fire burn much more quickly.
High concentrations of oxygen used during surgeries are a potential fire hazard for patients but that doesnt mean the O 2 gas itself catches. Oxygen by itself is not flammable. A flammable material is one that burns easily.
There is a risk of explosion and fire as pure oxygen is highly explosive and flammable. An explosion could occur when the percentage reaches a certain level. Instead an oxygen-rich environment causes everything within it to burn faster and hotter.
Of course now there is no smoking in hospitals. Oxygen is not flammable. To understand why you need to go into some detail Your lungs are basically a long series of tubes that branch out from your nose and mouth from trachea to bronchi to bronchioles and end in little thin-walled air sacs called alveoli.
The space agency countered that the risks of a fire in the crew cabin was minimal because it was a low-pressure environment. Oxygen does not burn. Many people believe that because oxygen is not flammable it does not pose much risk.
This definition does not apply to oxygen as it does not burn at all.
Hence is lighter than water. The density of water is around approximately 1 gram cubic centimetre 1 gcm 3.
Temperature Effects On Density
Ice is lighter and floats in.
Density of pure water. Density of solid water ie ice is lower than the density of liquid water at room temperature. Water pure weighs 1 gram per cubic centimeter or 1 000 kilogram per cubic meter ie. Density of a substance is defined as its mass per unit volume Mathematically Pure water has the maximum density at 4 0 C and minimum density at 0 0 C.
Thanks to Chuck Snelling The Expansion of Water at Various Temperatures. These two unusual properties allow water to moderate Earths. To find the density of water at 161 C you would first find the whole degree by searching down the left hand column until you reach 16Then you would slide across that row until you reach the column labeled 01.
At 25C 77F or 29815K at standard atmospheric pressure. It is used as a reference in most cases to determine the density and volumes of solids. Pure Water Density Standard UKAS ISOIEC17025 and ISO Guide 34 certified density.
Density of water pure is equal to 1 000 kgm³. In other words at the same temperature the density of water in gml or gcm 3 is 099777. Actually the exact density of water is not really 1 gml but rather a bit less very very little less at 09998395 gml at 40 Celsius 392 Fahrenheit.
When the temperature changes from either greater or less than 4 degrees the density will become less then 1 gcm 3. The density of water at 161C is 0998926 gmL. Seawater density is typically slightly higher than the density of pure water about 102gcm 3 to 103gcm 3.
The density of water varies according to temperature and the degree of purity. Waters density varies with temperature. T m 398 - 00225p-1.
Posted on January 14 2011 by Baby Pickel At 4C pure water has a density weight or mass of about 1 gcucm 1 gml 1 kglitre 1000 kgcum 1 tonnecum or 624 lbcuft At 4C pure water has a specific gravity of 1. It is temperature-dependent but this relation is said to be non- linear and also it is unimodal in nature rather than monotonic. In everyday life it is often assumed to approximate the water density to 1000 k g.
Other factors affect waters density such as whether it is tap or fresh water or salt water. The logic is to divide the value of kgm 3 by 1000 to get pure water density in gml. The maximum density of water occurs around 4 degrees Celsius.
Ice is less dense than liquid water so it floats. Density of pure water is a constant at a certain temperature not depending on sample. The value of the density of water is calculated with a formula in which the pressure and the temperature of the fluid are input parameters.
Purified water is water that has been mechanically filtered or processed to remove impurities and make it suitable for use. Water has its maximum density of 1gcm 3 at 4 degrees Celsius. The density of water reaches its maximum around 4C.
A standard table lists the values for the density of liquid water. Freezing water expands over 9 by volume and ice floats on water because it is lighter. Both below and above this temperature the density of water is lower.
At 4 degrees Celsius pure water has a density of 1gmL or 1kgL and a specific gravity of 1. 09970 gmL at 25 C. The density of the heavy water is 110595 g ml at 10 C and 110530 g ml at 20 C.
In Imperial or US customary measurement system the density is equal to 624 pound per cubic foot lbft³ or 058 ounce per cubic inch ozinch³. Water has a very high specific heat capacity of 41814 JkgK at 25 C the second-highest among all the heteroatomic species after ammonia as well as a high heat of vaporization 4065 kJmol or 2257 kJkg at the normal boiling point both of which are a result of the extensive hydrogen bonding between its molecules. 09982 gmL at 20 C density.
Note that water can be supercooled and remain a liquid well below its normal freezing point. Measurement of the Thermal Expansion of Pure Water. At the pressure of 101325 hPa ie.
The rounded value of 1 gml is what youll most often see though. That is why ice cubes float in water. The density of water is particularly important in metrology.
For example the rowcolumn shaded in yellow shows the density of pure water at 177C 0998650 gramscm 3 Density of Water gcm 3 at Temperatures from 0C liquid state to 309C by 01C inc. Water Density at Different Temperatures Below is a chart that shows the density of water in gramscm 3 at different temperatures ranging from below waters freezing point -22F-30C to its boiling point 212F100C. Find null-DENWAT MSDS related peer-reviewed papers technical documents similar products more at Sigma-Aldrich.
Water has the maximum density of 1 gcm 3 only when it is pure water. At room temperature ie 22 C the density of water in kgm 3 is 99777. Distilled water has been the most common form of purified water but in recent years water is more frequently purified by other processes including capacitive deionization reverse osmosis carbon filtering microfiltration ultrafiltration ultraviolet oxidation or.
In the so-called normal conditions the water density is equal to 1000 k g m 3 kgm3 k g m 3. Temperature tmat 0 C at which water has a maximum density at different pressurespin atm is determined by the formula. The density of pure water actually is somewhat less than 1 gcm 3.