Sodium Bicarbonate baking soda is a base it reacts with the acetic acid vinegar to produce water H2O and Sodium Acetate. The acidic compounds that induce this reaction include potassium bitartrate also known as cream of tartar lemon juice yogurt acetic acid vinegar etc.
 Nahco3 Hc2h3o2 Baking Soda And Vinegar Youtube
Nahco3 Hc2h3o2 Baking Soda And Vinegar Youtube
The reaction proceeds in two steps.
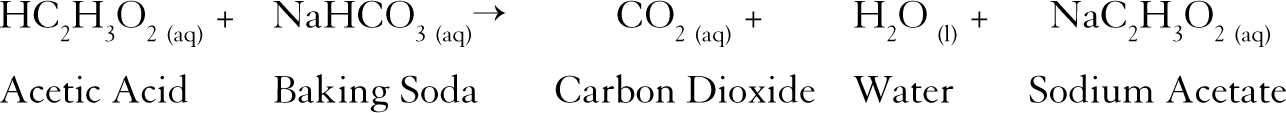
Sodium bicarbonate and acetic acid. Spray- wash treatments utilizing 10 acetic acid 3 hydrogen peroxide 1 sodium bicarbonate alone or in combination were performed to evaluate their efficacy in reducing numbers of Escherichia coli Listeria innocua andSalmonella wentworth. CO 2 turns lime water milky due to the formation of insoluble calcium carbonate and the milkiness disappears if excess of CO 2 is passed through the solution. The limiting factor for buffering lactic acid buildup in your muscles is the availability of sodium bicarbonate.
This helps dough to rise and gives it flavor and volume. In this experiment sodium bicarbonate was added to acetic acid which produces carbon dioxide liquid water sodium ions and acetate ions. The chemical equation for the overall reaction is.
Following equation summarizes the reaction between sodium bicarbonate and acetic acid. A chemical reaction occurs when acetic acid and sodium bicarbonate are combined in a closed film canister. Sodium bicarbonate IUPAC name.
The carbon dioxide released when sodium bicarbonate and acid react is responsible for some side effects of sodium bicarbonate use. Supplementing with sodium bicarbonate or baking soda increases the pH of the fluid outside your cells enabling your muscle cells to more readily remove lactic acid. In both the cases we can see that the metal ion sodium and calcium is dissociated from the anionic group and this cation reacts with the acetate group to form the respective salt.
Calculate the molar mass of sodium bicarbonate. Sodium bicarbonate is more commonly known as baking soda. Sodium bicarbonate can neutralize stomach acids offering quick but brief relief from indigestion.
In the process it also evolves CO2 carbon dioxide as a product. Spray-wash treatments utilizing 10 acetic acid 3 hydrogen peroxide 1 sodium bicarbonate alone or in combination were performed to evaluate their efficacy in reducing numbers of Escherichia coli Listeriainnocua and Salmonella wentworth. In a reaction between pure acetic acid and sodium bicarbonate 144 grams of sodium bicarbonate was added to 240 mL of acetic acid.
HC2H3O2 NaHCO3 -- NaC2H3O2 CO2 H2O The sodium bicarbonate and acetic acid react in a 11 ratio. Acetic acid reacts with sodium bicarbonate and gives brisk effervescence due to the formation of CO 2 gas. Sodium bicarbonate in gastronomy Sodium bicarbonate is primarily used in baking where it reacts with other components to release CO2.
CH3COOH acetic acid NaHCO3 sodium bicarbonate - NaCH3COO- sodium acetate H2CO3 The H2CO3 is not stable and breaks down quickly to form water and. Sodium hydrogen carbonate commonly known as baking soda or bicarbonate of soda is a chemical compound with the formula NaHCO 3It is a salt composed of a sodium cation Na and a bicarbonate anion HCO 3 Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powderIt has a slightly salty alkaline taste resembling that of. The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions.
Which reactant is the limiting reactant in this reaction. These include burping and gas or a feeling of being bloated in the stomach or abdomen. NaHCO 3 s CH 3 COOH l CO 2 g H 2 O l Na aq CH 3 COO - aq Materials 4 g of NaHCO 3 50 30 20 10 mL Vinegar with 5 VV Acetic Acid.
Rewrite the balanced chemical equation for the reaction between sodium bicarbonate and acetic acid. Calculate the molar mass of sodium.