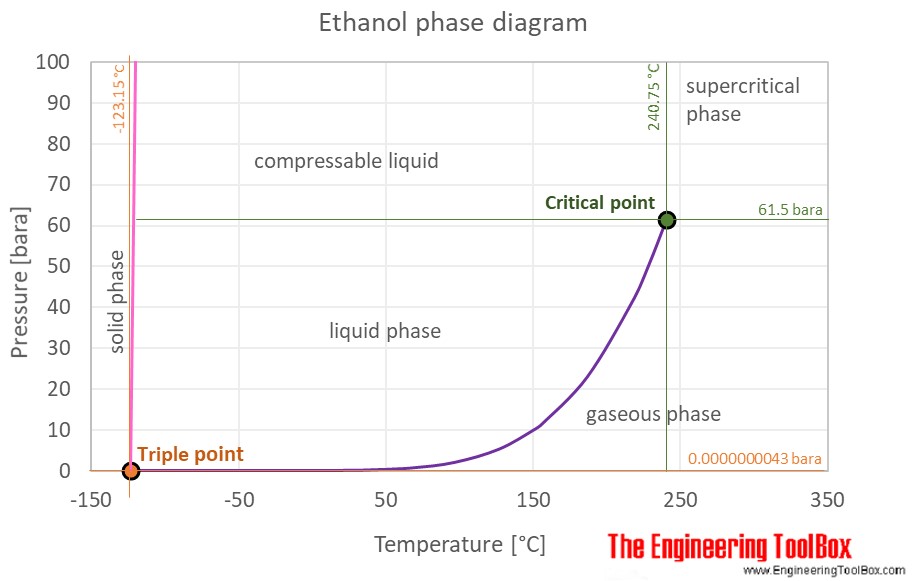
514 K 241 C 63 bar Std enthalpy change of fusion D fus H o 49 kJmol Std entropy change of fusion D fus S o 31 JmolK Std enthalpy change of vaporization D vap H o 3856 kJmol Std entropy change of vaporization D vap S o. Density 65 lb gal.
 The Vapor Pressure Of Ethanol Vs The Normal Boiling Point Temperature Download Scientific Diagram
The Vapor Pressure Of Ethanol Vs The Normal Boiling Point Temperature Download Scientific Diagram
The boiling point of pure ethanol C₂H₅OH MM 4607 gmol is 784 C.
What is the boiling point of ethanol. Melting point -114 C. 1 g of alcohol 7 Calories. For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F.
It is simple alcohol with the chemical formula. Vapors are heavier than air. Although dipole-dipole forces and London dispersion forces also exist between ethyl alcohol molecules the strong hydrogen bonding interactions are responsible for the much higher normal boiling point compared to methyl ether.
3732 K Boiling point of ethanol. A solution is made using 1000 g of ethanol the solvent and 271 g of glucose MM 18016 gmol. The melting point of this alcohol is -1141 o C and the boiling point is 785 o C.
2120 2089 2061 2030 Boiling Point of Ethanol. Cannabis oil extraction methods often use ethanol as an extraction solvent 59 and also as a post-processing solvent to remove oils waxes and chlorophyll from. On the other hand methoxymethane does not undergo H-bonding.
Boiling point 785 C. The boiling point of ethanol or grain alcohol C 2 H 5 OH at atmospheric pressure 147 psia 1 bar absolute is 1731 F 7837 C. Furthermore ethanol is a flammable liquid.
As ethanol also has a low boiling point it is easy to remove from a solution that has been used to dissolve other compounds making it a popular extracting agent for botanical oils. 10967 JmolK Molal freezing point constant. How many grams of chlorophyll C55H72MgN4O5 8935 gmol must be dissolved in 2900 grams of ethanol to raise the boiling point by 0350 C.
7837 C 1731 F Boiling point of methanol. Ethanol is an organic chemical compound. The boiling point of this mixture is 782C compared with the boiling point of pure ethanol at 785C and water at 100C.
Soluble in water not in fats. Ethanol is also called ethyl alcohol grain alcohol drinking alcohol spirits. Extra energy is required to break these hydrogen bonds.
What is the boiling point of this solution in C. 100 C 212 F Boiling point of water in Kelvin. Ethanol is a simple alcohol with the molecular formula C 2 H 5 OH.
1000 983 967 950 Boiling Point of Water. A nonvolatile nonelectrolyte that dissolves in ethanol is chlorophyll. Methanol methyl alcohol wood alcohol.
1731 1706 1681 1656 Atmospheric Pressure. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. Apart from that ethanol is polar due to the electronegativity difference between the oxygen and hydrogen in the OH group.
Hence the boiling point of ethanol is higher than that of methoxymethane. The normal boiling point of ethyl alcohol is 785 o C ie a liquid at room temperature. It is a clear colourless liquid with a characteristic odour.
Boiling point of water. Ethanol appears as a clear colorless liquid with a characteristic vinous odor and pungent taste. Chemical Structure of Ethanol.
In addition it can form hydrogen bonds due to the presence of OH group. 56 C 1328 F Boiling point of alcohol. Because the azeotropic mixture boils at a lower temperature its impossible to use simple distillation to produce ethanol at concentrations higher than 956.
Boiling point of ethanol is greater than that of ethane because there exists hydrogen bonding in alcohol molecules. The melting point of this alcohol is -1141 o C and the boiling point is 785 o C. 784 770 756 742 Boiling Point of Ethanol.
Ethanol is a polar compound. 647 C 1485 F Boiling point of acetone. The boiling point of a mixture of 956 ethanol by weight with 44 water is 781 C which is lower than the boiling point of pure water 100 C or pure ethanol 784 C.
Also due to OH group it has the ability to form hydrogen bonds. Density 0789 gmL. Ethanol undergoes intermolecular H-bonding due to the presence of -OH group resulting in the association of molecules.
Kb of ethanol is 122 Cm. 150 K 123 C 000043 Pa Critical point. 7837 C 1731 F Boiling point of nitrogen.
Due to which they have high boiling points. Due to this reasonethanol is liquid and ethane is gas. Boiling Point of Water.
On the other handthere is no hydrogen bonding in ethane thats why they have low boiling point. The boiling point of ethanol CH3CH2OH is 7850C at 1 atmosphere. Boiling points of common materials.
Ethanol reacts with strong oxidizing agents such as H KMnO 4 H K 2 CrO 4 H K 2 Cr 2 O 7 to give ethanoic acid. The chemical formula for ethanol is CH 3 CH 2 OH or C 2 H 5 OH condensed structural formulas The molecular formula for ethanol is C 2 H 6 O and its molar mass is 46068 gmol.
Ethanol Formula Boiling Melting Point Ph Density Solubility
Direct link to this balanced equation.
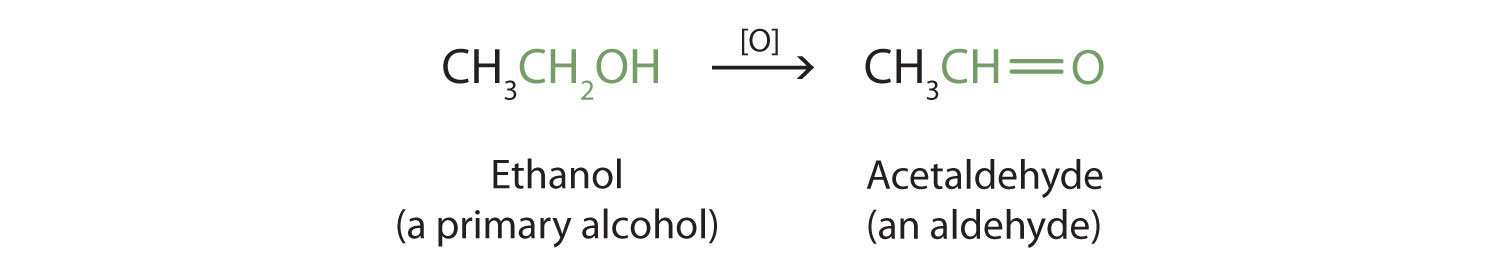
Chemical equation for ethanol. The molecular formula for ethanol is CH 3 CH 2 OH or C 2 H 5 OH. Ethanoic acid reacts with LiAlH 4 ether to give ethanol. Ethanol and ethanoic acid chemical properties.
Ethanoic acid to ethanol reaction. Ethanol is a chemical compound made up of carbon hydrogen and oxygen atoms. Ethanol is a clear colourless liquid with a smell that is referred to as the characteristic alcoholic odour.
This chemical formula can also be written as CH 3 CH 2 OH or C 2 H 5 OH. In chemistry terms ethanol is an alcohol also referred to as ethyl alcohol or drinking alcoholAs shown in figure 1 ethanol has the chemical formula Ethanol can be used as a fuel since it can produce heat energy as a result of alcohol combustion. The answer will appear below.
The chemical formula of ethanol is CH_3CH_2OH. Back in Lesson 2 I included a chemistry tutorial on some of the basic constituents of fuels. Ethanol is an alcohol compound.
Yeast is a single cell organism and a type of fungi. Ethanol also called ethyl alcohol grain alcohol or alcohol a member of a class of organic compounds that are given the general name alcohol s. A chemical reaction called fermentation takes place in which the glucose is broken down to ethanol by the action of enzymes in the yeast.
Only 5 of the ethene is converted into ethanol at each pass through the reactor. Five years later Archibald Scott Couper published the structural formula of ethanol. Pure ethanol is used as solvent for esters medicines solvent based paints and perfumes.
Ethanol is a compound of carbon hydrogen and oxygen elements was described by Antoine Lavoisier and its chemical formula was determined by Nicolas-Theodore de Saussure in 1808. It is a simple alcohol with the chemical formula C 2 H 6 O. Therefore both compounds have different chemical properties.
The molecular formula describes the type and number of atoms of elements present in an ethanol molecule. You may see ethanol written as EtOH where the Et represents the ethyl group C 2 H 5. C 2 H 6 Oethanol 3 O 2 2 CO 2 3 H 2 O Reaction type.
The chemical equation of the oxidation of ethanol with chromic acid Notice that ethanol has only one carbon-oxygen bond but in the product there is a new oxygen atom and a new carbon-oxygen. Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOH. Ethanol is a very important industrial chemical which is used as a solvent in the synthesis of other organic chemicals and as an additive to gasoline in the automotive industry forming a mixture called gasohol.
In this lesson we will be discussing the production of ethanol CH 3-CH 2-OH and butanol CH 3-CH 2-CH 2-CH 2-OH from starch and sugarEthanol or ethyl alcohol is a chemical that is volatile colorless and flammable. It is also used as a cleaning product methylated spirits. The chemical formula also may be written as CH 3 CH 2 OH.
Instructions on balancing chemical equations. Ethanol CH3CH2OH or C2H6O CID 702 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Ethanol is also the key component in almost all alcoholic drinks such as beer wine and spirits.
The reaction is reversible and the formation of the ethanol is exothermic. The equation for the reaction is. The empirical formula of ethanol is C 2 H 6 O.
Ethanols chemical formula is C 2 H 6 O. Ethanol is manufactured by reacting ethene with steam. Enter an equation of a chemical reaction and click Balance.
Ethanoic acid is carboxylic acid. Ethanol also called ethyl alcohol grain alcohol drinking alcohol spirits or simply alcohol is an organic chemical compound. Its molecular formula is C 2 H 5 OH.
The formula for ethyl alcohol or ethanol is C 2 H 5 OH or CH 3 CH 2 OH. 71 Ethanol Production - General Information. The shorthand formula is simply EtOH which describes the ethane backbone with a hydroxyl group.
By removing the ethanol from the equilibrium mixture and recycling the ethene it is possible to achieve an overall 95 conversion. It is made of nine atoms that include two carbon C atoms six hydrogen H atoms. Combustion Please tell about this free chemistry software to your friends.