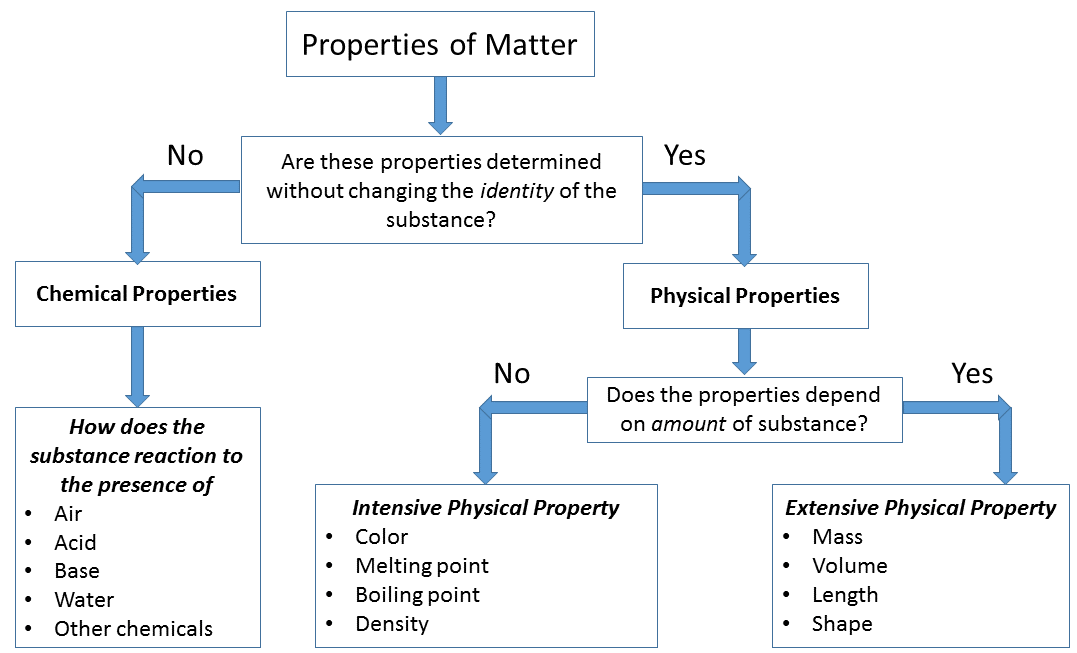
Chemical and Physical Changes. Any characteristic that can be determined without changing the substances chemical identity.
Physical And Chemical Properties And Changes Lessons Blendspace
Now let us discuss Benzene physical and chemical properties.
What are physical and chemical properties. The temperature at. This is due to the small size of boron which makes the sum of its first three ionization enthalpies very high. Mercury and gallium are metals but they are in liquid state at room temperature.
Aluminum is a bright lustrous metal widely obtained from nature. Sodium aluminum potassium magnesium. While moving from B to Al the sum of the first three enthalpies drastically decreases.
Physical properties of materials and systems are often described as intensive and extensive properties. This will include reactions such as combustion substitution addition hydration etc. Physical properties are properties that can be measured or observed without changing the chemical nature of the substance.
Physical Properties and Changes Physical properties can be observed or measured without changing the composition of matter. Benzene is not miscible in water and is soluble in organic solvents. A physical property is a characteristic of matter that is not associated with a change in its chemical composition.
The first three alkenes are gases and the next fourteen are liquids. Boron generally forms covalent bonds rather than 3 ions. If you see signs of a chemical reaction the characteristic you are measuring is most likely a chemical property.
In chemistry these properties are called Physical properties and Chemical properties. Physical Chemical Properties and Changes. All substances have distinct physical and chemical properties and may undergo physical or chemical changes.
Alkenes higher than these are all solids. These include bubbling color change temperature change and precipitation formation. If these signs are absent the characteristic is probably a physical property.
Physical Vs Chemical Properties. Familiar examples of physical properties include density color hardness melting and boiling points and electrical conductivity. Any characteristic that can be determined only by changing a substances molecular structure.
Let us take a look at few physical properties. Properties are used to identify elements. Physical properties such as hardness and boiling point and physical changes such as melting or freezing do not involve a change in the composition of matter.
Chemical properties such flammability and acidity and chemical changes such as rusting involve production of matter that differs from that present beforehand. They have a quality of reflecting. Properties are the characteristics of a substance which distinguishes it from another substance.
Color intensive density intensive volume extensive mass extensive boiling point intensive. The substances internal structure must be affected for its chemical properties to be investigated. All substances have distinct physical and chemical properties and may undergo physical or chemical changes.
Chemical Properties of Boron Oxidation states and trends in chemical reactivity. Physical properties such as hardness and boiling point and physical changes such as melting or freezing do not involve a change in the composition of matter. There are exception to this.
Many chemical properties of soils centre round the soil reaction. After reading this blog post your concepts about the physical and chemical properties of hydrocarbons will be cleared up 100. As regards their nature some soils are neutral some are acidic and some basic.
These properties relate to density corrosion ductility etc. Alkenes exist naturally in all three states. The acidity alkalinity and neutrality of soils are described in terms of hydrogen ion concentrations or pH values.
Flammability and corrosionoxidation resistance are examples of chemical properties. Benzene is a colourless compound and the physical state of Benzene is liquid. Chemical properties such flammability and acidity and chemical changes such as rusting involve production of matter that differs from that present beforehand.
Physical Properties of Alkenes. Physical Properties of Benzene. In the chemical properties part I am going to cover the chemical reactions for Alkane Alkenes and Alcohols.
Chemical and Physical Properties of Matter. The difference between a physical and chemical property is straightforward until the phase of the material is considered. Science - 8th.
All properties of matter are either extensive or intensive and either physical or chemical. Chemical properties such flammability and acidity and chemical changes such as rusting involve production of matter that differs from that present beforehand. Most common substances exist as solids liquids and gases which have diverse physical and chemical properties.
Physical properties 1 Physical state - Metals are solids at room temperature eg. Matter can undergo physical and chemical changes called Phase Changes. Chemical and Physical Properties.
Physical properties such as hardness and boiling point and physical changes such as melting or freezing do not involve a change in the composition of matter. Benzene melts at 55 C and it boils at 801 C. Physical properties of alkenes are quite similar to those of alkanes.
Physical properties are used to observe and describe matter. Physical and Chemical Properties of Aluminum makes it quite useful in human life. 2 Luster Metals have a shining surface called luster when freshly prepared.
Properties that describe how a substance changes into a completely different substance are called chemical properties. The characteristics that enable us to distinguish one substance from another are called properties. Chemical properties cannot be determined just by viewing or touching the substance.
Some examples of physical properties are.
Extensive and intensive properties. Check out these examples of everyday physical properties from both categories.
 Physical Properties Anchor Posters For Bulletin Board Physical Properties Of Matter Properties Of Matter Matter Science
Physical Properties Anchor Posters For Bulletin Board Physical Properties Of Matter Properties Of Matter Matter Science
All samples of a pure substance have the same chemical and physical properties.
Physical properties of matter. In order for us to measure or observe them we do not need to change the composition of the substance. Solids liquids and gases. Examples of Physical Properties of Matter There are many types of physical properties.
An unbalanced force can only change the state of motion of the matter. Examples of intensive properties include temperature refractive index density and hardness of an object. Physical changes are related to physical properties since some measurements require that changes be made.
It also has properties that we can describe through density solubility conductivity magnetism etc. It is a bulk property. This is an extensive list of physical properties of matter.
Unlike chemical properties you do not need to change the nature of a substance to measure any physical property it might have. Some physical properties are mass shape size volume color texture magnetism and conductivity. They include its flammability and susceptibility to corrosion.
Physical properties of matter are properties that can be measured or observed without matter changing to an entirely different substance. You can observe its mass by feeling how heavy it is when you try to pick it up. Everything you can see and touch is made up of matter.
Common Physical Properties A physical property is an attribute of matter that can be observed or perceived. Rodniqua BarrettWemyss Bight PrimaryNorth Eleuthera. Commonly used examples include density color odor hardness and volume.
Mass and volume are both examples of extensive physical properties. The property does not change with the size of a system. Physical properties are typically things you can detect with your senses.
There are two main types of physical properties. It is the resistance of the matter to change its state of motion. Chemical properties describe the characteristic ability of a substance to react to form new substances.
All matter has certain properties that define it. Two types of physical properties. You can observe its volume by looking at it and noticing its size.
Melting PointAs solid matter is heated it eventually melts or changes into a liquid state at the melting point. Matter exists in three main forms. Mass length and volume.
Physical properties are further. Depend on the amount of matter present. All matter has properties that allow us to describe.
Try Kids Academy with 3-day FREE TRIAL. For example they may be things that you can see hear smell or feel. The ability of a substance to change to a different substance.
These do not depend on the amount of matter. Inertia is one of the properties of matter. There are six major physical properties.
Melting point density colour conductivity boiling point etc. Physical properties are features that we can observe or measure without changing the object. The physical properties of matter are any properties that can be perceived or observed without changing the chemical identity of the sample.
These are characteristics that you can observe and measure without altering a sample. The six physical properties are color density volume mass boiling point and melting point. Mass and volume are both examples of extensive physical properties.
You can observe its mass by feeling how heavy it is when you try to pick it up. Physical properties Matter has mass and volume as demonstrated by this concrete block. Thousands of parents and educators are turning to the kids learning app that makes real learning truly fun.
Each material or object has a group of properties that make it different from others. An intensive property is a bulk property meaning that it is a physical property of a system that does not depend on the system size or the amount of material in the system. A property of a substance or system is called intensive when it is independent of the amount of the substance.
In contrast chemical properties are those that can only be observed and measured by performing a chemical reaction thus changing the molecular structure of the sample. Physical properties can often be seen. Physical properties are characteristics that scientists can measure without changing the composition of the sample under study such as mass color and volume the amount of space occupied by a sample.
Matter has mass and volume as demonstrated by this concrete block. Intensive properties are the same for 1 gram of a substance or 1 kilogram 1 cm 3 or 1 m 3. Matter is anything that has weight and takes up space.
You can observe its volume by looking at it and noticing its size.
As their name suggests nonmetals generally have properties that are very different from the properties of metals. Most nonmetals do not react with air in room temperature.
 3 Determine The Number Of Protons Neutrons And Electrons And The Mass Of An Element Using The Periodic Table Locating Metals Nonmetals Metalloids Ppt Download
3 Determine The Number Of Protons Neutrons And Electrons And The Mass Of An Element Using The Periodic Table Locating Metals Nonmetals Metalloids Ppt Download
One of the primary characteristics of nonmetals is that they form chemical compounds by making covalent and ionic bonds.

Most nonmetals have the properties of. They generally do not have lusture. As their name suggests nonmetals generally have properties that are very different from the properties of metals. Nonmetals are located to the far right of the periodic table therefore they must have high ionization energy.
Metals nonmetals and metalloids worksheet. Most nonmetals have the ability to gain electrons easily. They are generally poor conductors of heat and electricitySolid nonmetals are generally brittle with little or no metallic.
Properties of nonmetals include a relatively low boiling point which explains why many of them are gases at room temperature. Characteristic properties of non-metals are high ionization energies and high electronegativity. Non-metals are glasses or solids at room temp.
Carbon graphite is a non-metal but possesses high melting point. Owing to these properties non-metals usually gain electrons when reacting with other compounds forming covalent bonds. All non-metals except graphite posses low melting and boiling points.
They are generally poor conductors of heat and electricity. The most common chemical property is the type of oxide that the element forms. D none of the above.
B ability to conduct heat. They are bad conductors of heat and electricity. Following are the important chemical reactions of non-metals.
Most metals have high tensile strength. Except for Chlorine chlorine. Nonmetals have high ionization energies and electro negativities.
Most or some elements in each category share a range of other properties. Choose the nonmetallic elements from the list. Usually nonmetals do not react with water.
5 Properties Of Nonmetals. Gain electrons instead of giving them up. Nonmetals have high ionization energies and electronegativities.
Metals and non-metals can also be distinguished by some chemical properties. Are poor conductors of heat and electricity. Rank the nonmetals in each set from most reactive 1 to least reactive 3.
Ionization energy is the amount of energy required to remove an electron from its. Properties of nonmetals include a relatively low boiling point which explains why many of them are gases at room temperature. Properties of Nonmetals.
Halogen Nonmetal only Noble gas Halogen Nonmetal only. However some nonmetals are solids at room temperature including the three pictured above and one nonmetal - bromine - is a liquid at room temperature. Sulfur nitrogen selenium oxygen carbon.
These will have the mixed properties of both metals and nonmetals. Metals form oxides that are basic but. Elements related under nonmetals are sulfur carbon phosphorus all halogens hydrogen oxygen nitrogen selenium and noble gases.
Typical nonmetals have a dull coloured or colourless appearance. Why are nonmetals unable to carry an electric current. Properties of nonmetals include a high boiling point.
Check all that apply. Teks 6 6a compare metals nonmetals and metalloids using physical properties such as luster conductivity and malleability. Metas and nonmetals g7.
Non-metals are brittle they are neither malleable nor ductile. Among the non-metals the anionic dopants have a strong influence on the VB. Usually nonmetals react with other nonmetals in high temperature.
These are located on the right-hand side of the periodic table. Which nonmetals have similar chemical properties. Nonmetals are very few numbers in the periodic table are.
They are not as hard as metals. Chemical properties of nonmetals Non-metals are also called electronegative elements because the non-metal atom form negatively charged ion by accepting electrons. All nonmetals are very reactive.
Are brittle when solid. Nonmetals react more with metals than with nonmetals. Non-metals are non-lustrous and cannot be polished Non-metals may be solid liquid or Gases at room temperature Non-metals have low melting and boiling points as compared to metals Most of solid metals are soft Non-metals are not strong Non-metals are light substances Non-metals are non-sonorous.
Solid nonmetals are generally brittle with little or no metallic luster. However some nonmetals are solids at room temperature including the three pictured above and one nonmetal - bromine - is a liquid at room temperature. Nonmetals display a wide range of chemical properties and reactivities.
And have acidic oxides. A few elements have properties that are either anomalous given their category or otherwise extraordinary. White phosphorus is the only nonmetal that reacts with air to form its oxide by burning.
Metalloids have properties between metals conductors and nonmetals insulators and so they can conduct electricity but not very well. In covalent bonds two elements will share valence electrons until they have a complete shell.
Soluble in carbon disulfide. The physical properties are as follows.
 Elements A Closer Look Ppt Download
Elements A Closer Look Ppt Download
White phosphorus is a poisonous waxy solid and contact with skin can cause severe burns.

Physical properties of phosphorus. The melting point of phosphorus white is 441C boiling point white is 280C specific gravity white is 182 red 220 black 225-269 with a valence of 3 or 5. We observed that the growth pattern of Al deposited onto the BP film is. The white form sometimes called yellow phosphorus is similar to wax the red and purple forms are non-crystalline solids while the black allotrope is equivalent to graphite in pencil lead.
The Physical properties of Phosphorus are the characteristics that can be observed without changing the substance into another substance. It is insoluble in water. Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure.
It contains a phosphorus dimer as a structural unit and is highly reactive. It can be sliced with a knife. Upon heating to temperatures above 300 o C red phosphorus undergoes crystallization.
Particularly motivated by the study of graphene and other two-dimensional 2D materials many forms of phosphorus have been revisited from the perspective of 2D materials different from its early studies mostly as a bulk material. It is colorless or white. Another allotrope is diphosphorus.
The annual production of elemental phosphorus is around 1000000 tons. White phosphorus is yellowish white solid that has a waxy texture. Physical properties are observed using senses like colour boiling point freezing point melting point hardness density and odour.
It is not poisonous to humans in contrast to the white phosphorus allotrope. It is found in nature in several allotropic forms and is an essential element for the life of organisms. Physical properties are usually those that can be observed using our senses such as color luster freezing point boiling point melting point density hardness and odor.
Its melting point is 441C 111F and its boiling point is 280C 536F. White Phosphorus and Related Molecular Forms. Some important physical and chemical properties of red phosphorus are tabulated below.
There are four allotropic forms of phosphorus. Two forms of white or yellow red and black or violet. Physical Properties of Phosphorus.
Phosphorus has unique structures and properties which lead to a variety of different applications. Red phosphorus is odourless and has a deep red colour. Ordinary phosphorus or white phosphorus is a soft solid.
Insoluble in water and ethyl alcohol. This new perspective motivates exploration of phosphorus to be. But due to the effect of light its color gradually turns yellow.
It glows in the dark and is spontaneously flammable when exposed to air. The electronic properties of the interface between Al and black phosphorus were studied by photoemission spectroscopy PES. Its melting point is 44 0 C and the boiling point is 287 0 C.
Phosphorous is a multivalent nonmetal of the nitrogen group. The volume which is occupied by the atom of phosphorus when it is combined with one or two other elements to form liquid compounds has been deduced from the molar volumes of such compounds on the assumption that the other elements possess constant and characteristic atomic volumes in these compounds. There are several forms of phosphorous called white red and black phosphorous although the their colours are more likely to be slightly different.
The two main forms of phosphorus are white phosphorus and red phosphorus. The fluorescence intensity of phosphorus corroles increases upon meso-aryl CFCH and POHPF substitutions the latter affects corrole-centered redox processes more than CHCF substitution on the corroles skeleton and the presence of F atoms allows for the first experimental insight into the electronic structures of oxidized corroles. Physical Properties of Phosphorus The Physical properties of Phosphorus are the characteristics that can be observed without changing the substance into another substance.
These allotropes all have different physical and chemical properties. It smells like garlic. Phosphorus undergoes spontaneous ignition in air and forms pentoxide P 4 O 10.
White phosphorus is a waxy transparent solid. Physical properties are usually those that can be observed using our senses such as color luster freezing point boiling point melting point density hardness and odor. Physical Properties of Phosphorus Physical properties are the characteristics that can be seen without changing the substance into another substance.
In appearance properties and structure black phosphorus resembles graphite it is black and flaky a conductor of electricity and has puckered sheets of linked atoms. Phosphorus is a poor conductor of heat and electricity except for black phosphorus. There are two allotropic forms of phosphorus red and black that differ in physical and chemical properties.
All types of phosphorus are stable at room temperature. 321 Physical Description Phosphorus white dry or under water or in solution appears as a soft waxy solid with a sharp pungent odor similar to garlic.