Therefore they will have the same type of bonding and forces influencing them. Water has a very high specific heat capacity of 41814 JkgK at 25 C the second-highest among all the heteroatomic species after ammonia as well as a high heat of vaporization 4065 kJmol or 2257 kJkg at the normal boiling point both of which are a result of the extensive hydrogen bonding between its molecules.
Therefore the boiling point of H₂S should be less than 300K.
Boiling point of h20. Boiling point elevation equation. Therefore our next course would be looking at the molecular. MgBr2s H20 -- Mg2 aq 2Br- aq Chemistry.
This approximation is sufficient for this kind of calculations. 100 low impact 20 minute total body workout. This boiling point calculator finds the pressure at altitude assuming that the pressure at sea level is constant and equal to 1013 hPa 1013 bar.
The melting point and freezing point of water ideally are the same especially if there are gas bubbles in water but if the water is free of nucleating points water can supercool all the way down to 42 C 436 F 231 K before freezing. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33 Ice Water - Melting Points at Higher Pressure - Online calculator figures and tables showing melting points of ice to water at pressures ranging from 0 to 29000 psia 0 to 2000 bara. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
Therefore the correct option is 2 Prev Question Next Question Related questions 0 votes. And we aint got no data here. During boiling one has to break the bonds and hence greater amount of energy is required in case of H20 since it is more stable and thus it has a higher boiling point.
1 Greater than 300 K but less than 373 K. The boiling of water 373 K is abnormally high when compared to that of H2S 2112 K. Physical-chemistry intermolecular-forces hydrogen-bond phase.
You should look up the boiling points on the web or in your text and then you should try to rationalize them on the basis of intermolecular or interparticle force. Workout 5 of 5 H20 Plan. The boiling point of water depends on the atmospheric pressure which changes according to elevation.
H2O HF NH3 CH4. Which has the highest boiling point. H20HFNH3 in that order although I assume youre really looking for the explanations here.
So in some cases the melting point of water is considerably higher than its freezing point. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. The given substances according to their increasing boiling points can be arranged as.
Order of increasing boiling point stackrelrarrN_2 HCl H_2O NaCl But a scientist interrogates data. So what is an explanation for the difference in boiling point between hydrogen fluoride and ammonia. The boiling point of a liquid varies depending upon the surrounding environmental pressure.
Compare the amount of heat required to vaporize a 200-gram sample of H20L at its boiling point to the amount of heat required to melt a 200-gram sample ofH20s at its melting point. It is obvious that will have the highest boiling point as it has hydrogen bonding. The remaining compounds like have the same group in the periodic table.
Hydrogen bond is more strong than Van Der Waals ForcePresent in H2S so H20 is more stable. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. Water boils at a lower temperature as you gain altitude eg going higher on a mountain and boils at a higher temperature if you increase atmospheric pressure coming back down to sea level or going below it.
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. If the boiling point of H2O is 373 K the boiling point of H2S will be. H2O has the highest boiling point due to its ability to form 2 hydrogen bonds per molecule.
Write the equation for the dissolution of magnesium bromide in water. These two unusual properties allow water to moderate Earths. Remember that the boiling point of water depends solely on pressure.
Boiling point of fluoride is 195 degrees Celsius while boiling point of ammonia is minus 33 degrees Celsius which makes 535 degrees difference.
You dont have to use our boiling point at altitude calculator to determine the boiling point of water at sea level. According to Table PageIndex1 the molal boiling point elevation constant for water is 051Cm.
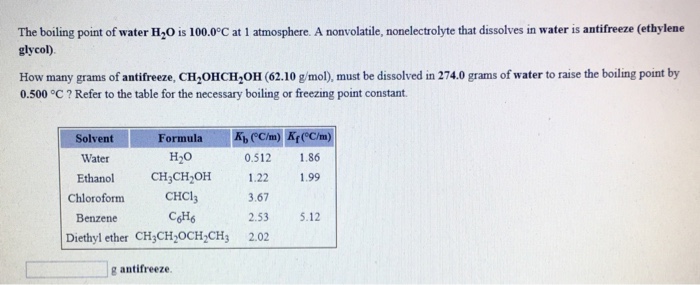
 Water Boiling Points At Vacuum Pressure
Water Boiling Points At Vacuum Pressure
Pressure must be within the ranges 1-220 bara 147-3200 psia 760-165 000 mm Hg or 30-6500 in Hg.
/BoilingWater-58dd1c2a5f9b5846837d2a23.jpg)
Boiling point of h2o. Water boils at a lower temperature as you gain altitude eg going higher on a mountain and boils at a higher temperature if you increase atmospheric pressure coming back down to sea level or going below it. The normal boiling point is 9997 C 2119 F at a pressure of 1 atm ie 101325 kPa. One other problem is that of boiling water for cooking food.
Actually the formula for boiling point uses this value as the basis of calculations. It is always the same - 100C or 212F. The boiling point of a liquid varies depending upon the surrounding pressure.
The calculator below can be used to calculate the water boiling point at given absolute pressures. Because of this comparatively weak intermolecular forces exist for H2S and the melting and boiling points are much lower than they are in water. Liquid helium has the lowest boiling point of all about -452 degrees Fahrenheit only 42 degrees Celsius above absolute zero.
It varies from 72C to 101C accordingly from the highest point to the lowest point on land. There are two conventions regarding the standard boiling point of water. The boiling point of water at standard atmospheric pressure is 212oF 100oC.
The boiling point of a liquid varies according to the applied pressure. Boiling points of water at absolute pressures ranging from 1 to 70 bara or 147 to 1000 psia are indicated in the figures and tables below. Since 1 Kg or 1000 g of water has a volume of 1 L or 1000 mL then 1000 mL of water has a mass of 1000 g.
The normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure 760 mm 2992 inches of mercury. The other molecules are slightly polar and show the increase in boiling point with molecular weight which is normal. As defined earlier atmospheric pressure or air pressure is the force applied against a liquids surface.
A lower boiling point means that food cooks at a lower temperature despite the fact that the water is boiling. What is the boiling point of h2s-60 C. See Water and Heavy Water for thermodynamic properties at standard condtions.
Online Water Boiling Point Calculator. The boiling point of water at atmospheric pressure 101315 Pa is 100c however the boiling point is changing with pressure this page is giving table and abacus to know whatis the boiling point of water at different pressures. Thus a 100 m aqueous solution of a nonvolatile molecular solute such as glucose or sucrose will have an increase in boiling point of 051C to give a boiling point of 10051C at 100 atm.
H2Te has the highest molecular weight and thus it has the highest boiling point from H2S and H2Se. The boiling point of water varies at various locations. It is important to recognize just how much the temperature of boiling water is reduced as the altitude increases.
Nitrogen N2 carbon dioxide oxygen O2 helium chlorine Cl2 and hydrogen are all familiar examples of substances that boil at much lower temperatures than water. Although water boils at 100C at sea level the boiling point on top of Mount Everest is only about 70C. Different substances have different boiling points a main reference to boiling points is the boiling point of water H2O which is 100 degrees Celsius Why is the boiling point of h2o higher than.
The boiling point of the solvent water depends on the number of dissolved particles in every kilogram of solvent water in this case. You may have heard that water always boils at 100C but this is not completely true. The boiling point of water depends on the atmospheric pressure which changes according to elevation.
The IUPAC recommended standard boiling point of water at a standard pressure of 100 kPa 1 bar is 9961 C 2113 F. At sea level water boils at 100 C 212 F. As a thumb rule the higher the atmospheric pressure the more heat energy is necessary to boil a liquidor in this case water.
Boiling point of water at sea level. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. At higher altitudes the temperature of the boiling point is.
The oxygen content of the air is much lower than at sea level making it necessary to bring oxygen tanks along although a few climbers have reached the peak without oxygen. Instead water boils at 100 C which is very abnormal. Boiling point of Water.
If water behaved as a normal polar molecule it would have boiled at about - 100 C shown in red. Likewise which has the highest boiling point h2o h2s or H2Se. The reason for these variations is the lowering of atmospheric pressure as we travel.
Since it is not advisable to boil water in order to remove fluoride lets look at some methods that can actually work. Resulting in the same amount of fluoride for a smaller amount of water.
 Does Boiling Water Remove Fluoride Tested With Meter
Does Boiling Water Remove Fluoride Tested With Meter
If anything it does the exact opposite it increases the concentration of fluoride in the water.

Does boiling water remove fluoride. Decreasing the amount of fluoride is a good step to ensure good health so you can start investing in the best water distiller. You now know that distillers use boiling evaporation and condensation to produce the best-quality fluoride-free water. Boiling water may cut out the basic contaminants but it cannot deal with salts and chemicals like fluoride easily.
Since boiling is not advised to remove fluoride from water lets take a closer look at some of the methods that can be effective. Boiling water does not remove fluoride from water. This is where you boil water and collect the vapor in another container.
Excessive amounts of fluoride consumption lead only to bad health issues. The water you collect will contain much less fluoride than your starting water. Actuality heating water to boiling will concentrate the fluoride rather than reduce it.
One of the most common questions Can You Remove Fluoride By Boiling Water The answer is no. No it does not Does boiling water remove fluoride. No you cannot remove fluoride from your tap water by boiling it.
In fact when you boil water it tends to concentrate the fluoride as water evaporates. The short answer is no. Boiling water will not only fail to remove fluoride but will actually have the opposite effect.
Similar to distillation RO has a good track record for removing almost everything from water. Because boiling water removes bacteria and other organisms some may mistakenly believe boiling tap water will also remove fluoride. However you can boil water to remove fluoride if you capture the water that is evaporated and then condense it.
When you boil water then fluoride will become more concentrated remaining in the water as a fluorine salt. Boiling in itself does not reduce even a small amount of fluorine from the water. In fact it can have a negative effect leading to the formation of a more potent form of fluoride known as fluorine salt.
Boiling tap water is well known for killing bacteria and dissolving gases like oxygen. There are some methods many people assume will remove fluoride from water but theyre actually quite ineffective. Reverse osmosis RO relies on pressure and a semi-permeable membrane to remove contaminants from water.
When buying a water filter you may be comforted by reading that the system you are purchasing removes 95 to 99 of contaminants but if it does not specifically state that it removes fluoride you can bet it doesnt. Thus actually increasing the amount of fluoride per volume of water. According to the fluoride meter boiling water does not remove fluoride.
The short answer to this question is that you cannot remove fluoride from drinking water by boiling. The most popular water filters the inexpensive activated-carbon pitchers and tap-attachments sold under the brand names Brita and Pur cant remove fluoride. The only way you can use boiling to remove fluoride from water is through the distillation process.
Most water filter sales literature avoids the subject. Sticking your water into the freezer will not affect the concentration of fluoride. RO can remove between 90 and 95 of fluoride depending on the efficiency of the system and on how well the system is maintained.
Boiling to Distill Water to Remove Fluoride. What Will Not Remove Fluoride from Water. It is not that its explicitly bad but It is not something you need to have in water as I believe with balanced diet we have enough of it from elsewhere.
A lot more than just boiling water has to be done to get rid of fluoride from water. Some people albeit a small number also believe that freezing water can help remove fluoride from the water. In fact boiling water can have an adverse effect- as it may increase the concentration of fluoride.
When water temperature hits its boiling point 212 degrees Fahrenheit water vapor escapes and leaves behind all dissolved solids and minerals. It will cause a more concentrated form of fluoride called fluorine salt. This is due to water evaporating and fluoride staying untouched.
As an example when you boil a pot of water on the stove the fluoride concentration in the water in the pot increases. Does Boiling Water Remove Fluoride. Fluoride is a kind of salt you cant remove fluoride by boiling water.
Yes as mentioned in the article unfortunately boiling does not remove fluoride out of water. However this method actually increases concentration because as the water boils the vapors escape and leave the mineral behind. While boiling water can remove some impurities from your water the process has been shown to increase the concentration of fluoride salt.
One of the most frequently asked question is on whether boiling tap water removes fluoride. So you may well be wondering whether you can perform your own distillation in your free time at home. As the volume of water decreases through boiling the fluoride concentration actually goes up.
Boiling your water wont help as the fluoride does not evaporate easily like chlorine. In the end boiling water does not remove fluoride from water. Boiling will actually have an adverse effect.
To top it all off fluoride is difficult to remove from water. Boiling does not help remove fluoride from water because fluoride does not evaporate easily.
Therefore the boiling point of a liquid depends on atmospheric pressure. The point of crisis.
 Difference Between Flash Point And Boiling Point Compare The Difference Between Similar Terms
Difference Between Flash Point And Boiling Point Compare The Difference Between Similar Terms
Hot gas from steam engine condensing.
Boiling point science definition. Boiling or the boiling point is the temperature when a liquid will have vaporization occurring through the entire liquid not just on the surface. The boiling point of a substance is the temperature at which it can change its state from a liquid to a gas throughout the bulk of the liquid. Mixturesmelt and boil over a rangeof temperatures.
For example water boils at. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A stricter definition of boiling point is the temperature at which the liquid and vapor gas phases of a substance can exist in equilibrium.
The boiling point for water is 100 degrees C 212 degrees F. As the temperature of a liquid rises the pressure of escaping vapor also rises and at the boiling point the pressure of the escaping vapor is equal to that exerted on the liquid by the surrounding air causing bubbles to form. The boiling point becomes lower as the external pressure is reduced.
Boiling is defined as a phase transition from the liquid state to the gas state usually occurring when a liquid is heated to its boiling point. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Two other words for boiling are ebullition and vaporization.
The boiling point of a pure substance is the temperature at which the substance transitions from a liquid to the gaseous phase. The boiling point is defined as the temperature at which a liquids saturated vapour pressure equals the atmospheric pressure surrounding it. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure.
At the boiling point the vapor pressure of the liquid is the same as the external pressure acting upon its surface. The temperature at which matter is converted from the liquid state to the gaseous state. Boiling points can be changed in several ways.
Under this condition addition of heat results in the transformation of the liquid into its vapour without raising the temperature. This occurs when the liquids vapor pressure. This is measured at one atmosphere that is the air pressure at sea level.
The point at which a person becomes uncontrollably angry. Boiling point definition the temperature at which the vapor pressure of a liquid is equal to the pressure of the atmosphere on the liquid equal to 212F 100C for water at sea level. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
Boiling Point Definition The temperature at which liquid vapour pressure equals atmospheric pressure is referred to as boiling point. Boiling point temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid. Boiling The temperature at which a liquid changes to a vapor or gas.
For pure water this is 100 Celsius or 212 Fahrenheit. The boiling point of a substance is the temperature at which the substance boils or enters a state of rapid evaporation. Puresubstances have specificmelting and boiling points.
The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The boiling point at a pressure of 1 atmosphere is called the normal boiling point. Boiling is the rapid vaporization of a liquid which occurs when a liquid is heated to its boiling point the temperature at which the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere.
Again at a certain temperature called the boiling point the molecules will gain enough energy to break free and become a gas. The temperature at which a liquid boils. Definition of boiling point.
Water at its boiling point. When heat is applied to a liquid the temperature of the liquid rises until the vapor pressure of the liquid equals the pressure of the surrounding gases. Boiling-point meaning The temperature at which the vapor pressure of a liquid is equal to the ambient atmospheric pressure.
A liquid may change to a gas at temperatures below the. When a liquid becomes a gas it is called boiling or vaporization. Boiling pointis the temperature at which a liquid changes into a gas.
Head sense 17b matters had reached the boiling point. At this point the vapor pressure of the liquid is equal to the applied pressure on the liquid.
610 m 208 oF. When the altitude increases the boiling point.
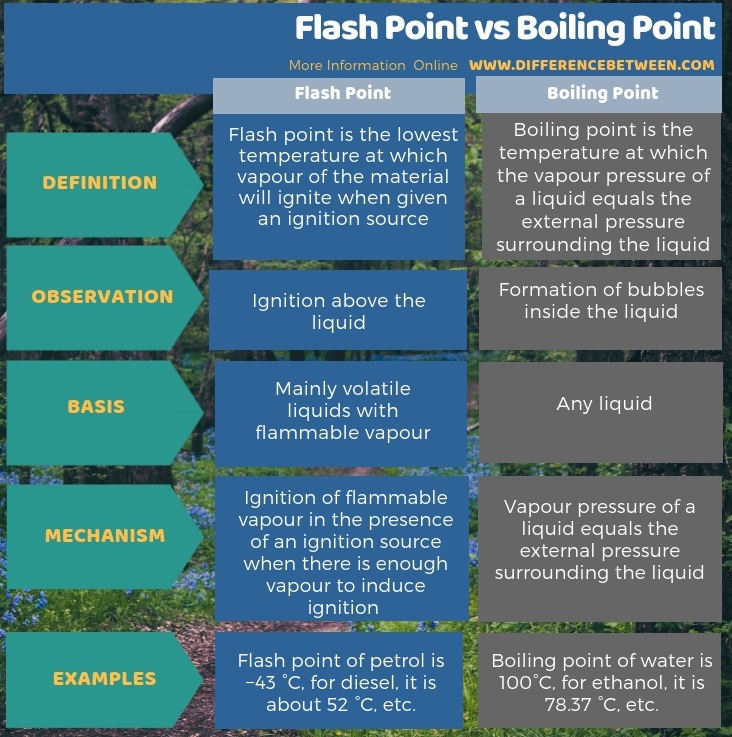
 Water Boiling Points At Higher Pressure
Water Boiling Points At Higher Pressure
See Water and Heavy Water for thermodynamic properties at standard condtions.

Boiling point of water. The boiling point of water depends on the atmospheric pressure which changes according to elevation. For pure water the boiling point is 100 degrees Celsius 212 Fahrenheit at one atmosphere of pressure and the melting point is 0 degrees Celsius 32 degrees Fahrenheit at one atmosphere of pressure. The IUPAC recommended standard boiling point of water at a standard pressure of 100 kPa 1 bar 7 is 9961 C 2113 F.
The boiling point of water at a pressure in mbar. Boiling Point of Water at 1 Atmosphere. At 1 atmosphere the boiling point of pure water that is water with no added minerals or salts is 100 degrees Celsius or 212 degrees Fahrenheit.
This video channel is developed by Amrita Universitys CREATEhttpwwwamritaeducreate For more Information httpamritaolabseduinsub73brch2si. The boiling point of water is 1000C or 2120F. The normal boiling point is 9997 C 2119 F at a pressure of 1 atm ie 101325 kPa.
However the value is not a constant. 457 m 209 oF. The boiling point of water can be higher or lower depending on several factors.
This is only at sea level. The boiling point of water is typically considered to be 100 C or 212 F. At higher altitudes the temperature of the boiling point is lower.
Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water at pressures ranging from 147 to 3200 psia 1 to 220 bara. Reference tables contain values of the boiling point of water at different pressures in different unit of measure. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure.
Altitude has an effect on the boiling point of water according to the Whats Cooking America website. Nitrogen boils at -196 C. 152 m 211 oF.
1 atmosphere is the standard atmospheric pressure at sea level. The temperature of water increases and at certain temperature it begins to boil. Conventionally the temperature at which water boils is 100 degrees Celsius or 212 Fahrenheit but only at sea level.
Simmering is gentle boiling while in poaching the cooking liquid moves but scarcely bubbles. Pressure can be measured in atmospheres. Boiling points of water at absolute pressures ranging from 1 to 70 bara or 147 to 1000 psia are indicated in the figures and tables below.
1000 ft 305 m 210 oF. There are two conventions regarding the standard boiling point of water. 762 m 207 oF.
This is a very high temperature that can scald the. Boiling is the method of cooking food in boiling water or other water-based liquids such as stock or milk. The boiling point of water is 100 C.
T - temperature C. P- pressure mbar bar torr. In this regard the boiling point of water changes with a change in barometric pressure.
0 m 212 oF. As a thumb rule the higher the atmospheric pressure the more heat energy is necessary to boil a liquidor in this case water. The boiling point of distilled water is 100 degrees Celsius or 212 degrees Fahrenheit.
As defined earlier atmospheric pressure or air pressure is the force applied against a liquids surface. An experiment to determine the boiling point of water. Developed by Amrita University Under research grant from Department Of Electronics Information Technology.
1219 m 204 oF. The boiling point of silver 2212 C. 3000 ft 914 m 206 oF.
When you take some water in a beaker and continue to heat it what happens. Pressure and a change in the composition of the liquid may alter the boiling point of the liquid. At sea level water boils at 100 C 212 F.
The boiling point of water at standard atmospheric pressure is 212oF 100oC. Boiling Point - Celsius. 1067 m 2055 oF.
Boiling water is characterized by energetic bubbles and steam and it is considered to be hot. Temperature given as C F K and R. The boiling point of a substance or water indicates that there is an increased temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid.
Boiling point of Water The boiling point of water is 100C or 212 F at 1 atmosphere of pressure sea level. At at high altitudes the lower pressure makes the boiling point several degrees lower. Boiling Point - Fahrenheit.
Neon boiles at -246 C.