Synthesis reactions and physical properties of stable mononuclear platinum and zirconium complexes of cyclohexyne reported prior to the late 1980s have been comprehensively covered in earlier reviews. The word synthesis means to put together.
 Synthesis Combination Reaction Definition Examples And Applications
Synthesis Combination Reaction Definition Examples And Applications
In a generic way the reaction can be represented as the form.

What is a synthesis reaction. What is a Synthesis Reaction. Please update your bookmarks accordingly. The same can be said for the decomposition reaction which acts in reverse to show what happens to complex compounds when they decompose to simpler elements.
Synthesis reactions are reactions that occur when two different atoms or molecules interact to form a different molecule or compound. Synthesis reactions release energy in the form of heat and light so they are exothermic. Sometimes synthesis reactions can result in the formation of more.
The synthesis reactions are very important for science because thanks to these methods can be made various materials medicines and products that we use in everyday life. A synthesis reaction is the joining together of two reactants or compounds to produce a complex product also called a compound. A synthesis reaction is a type of chemical reaction in which two or more components combine with each other to form a large compound.
A B C. The reaction of a metal with a non-metal to produce a compound is an example of a synthesis reaction. A synthesis reaction can occur when combining elements and producing a new compound combining compounds to produce a new compound or combining both elements and compounds to result in a new compound.
When two non-metals combine Frying an egg is a synthesis reaction. However an endothermic outcome is also possible. In chemistry a synthesis reaction is when two or more chemicals combine and form a more complex product.
A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Synthesis is in essence the reversal of a decomposition reaction. It is applied to all types of chemical compounds but most syntheses are of organic molecules.
Definition and example of a synthesis reaction. The substance could be elements or compounds but the synthetic product is always a compound. Synthesis Reaction Definition A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product.
Synthesis reaction Chemical synthesis the construction of complex chemical compounds from simpler ones. In a synthesis reaction two substances combine to produce a single product. When a metal and non-metal are combined they produce an ionic compound.
We can call it a direct combination reaction as well because it involves the combination of components to form a. A synthesis reaction is a type of chemical reaction in which two or more components combine with each other to form a large compound. It is the opposite of the dissociation reaction.
Synthesis reactions are those in which two or more compounds react to certain conditions to form one or more new products. Two water molecules to follow the above example evaporate into the products of one of oxygen atom and one hydrogen atom or 2H2O 2H2 O2. The synthesis reaction is the opposite of the dissociation reaction.
In a synthesis reaction two or more chemical species combine to form a more complex product. A synthesis reaction is a reaction in which two or more reactants combine to form a more complex substance. Most of the time when a synthesis reaction occurs energy is released and the reaction is exothermic.
A synthesis reaction is a type of reaction in which multiple reactants combine to form a single product. The synthesis reaction of water molecules is the easiest to visualize. An example of a synthesis reaction is the formation of water from hydrogen and oxygen.
It is the process by which many substances important to daily life are obtained. Synthesis reaction the reverse of the decomposition reaction also named direct combination reaction is one of the most common types of chemical reactions in which two or more substances combine to form a more complicated one. What is a Synthesis Reaction.
In this form a synthesis reaction is easy to recognize because you have more reactants than products. A B -- AB. A B AB This type of reaction is also called a direct combination reaction or simply a combination reaction.
The general chemical equation for a synthesis reaction is A B AB. The opposite of a synthesis reaction is a decomposition. We have moved all content for this concept to for better organization.
A B AB. It is also called a direct combination reaction as well because it involves the combination of components to form a new compound. You will also have more reactants than products since two or more chemical species combine to form one new larger compound.
28 More recently reaction of the zirconocene complex of cyclohexyne with trimethylaluminum and trimethylgallium has been reported to give 247 and 248 respectively Eq. The reactants may be elements or compounds while the product is always a compound. The synthesis reaction has the distinction of being one of the easiest to visualize with the simplest equation to balance.
A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Its the type of reaction that forms compounds from their elements.
In this science activity children will explore chemical reactions with baking soda and vinegar. Baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion.
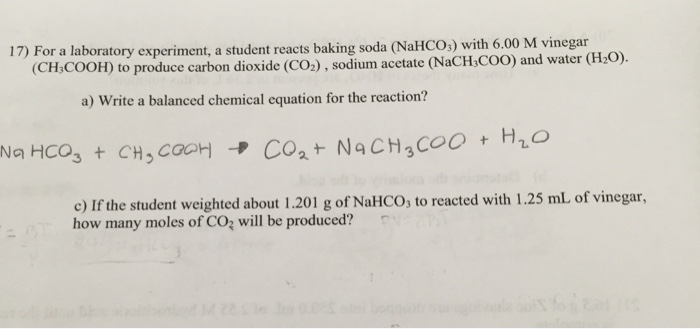
Baking Soda and Vinegar Reaction Equation Baking Soda Vinegar - Carbon Dioxide Water Sodium Ion Acetate Ion You can read more about the chemistry behind the reaction here.
Baking soda and vinegar reaction equation. Refill the vinegar jars and pour in more baking soda and they are ready to go again. Baking Soda and Vinegar Rocket. The equation for the reaction is.
The first reaction is the acid -base reaction. The balanced chemical equation is. Frozen vinegar room temperature baking soda cold baking soda room temperature vinegar 1 tsp vinegar 12 tsp baking soda 1 tsp baking soda 12 tsp vinegar put the baking soda down first Any way lets go to step two.
If youre wondering if a chemical reaction is happening look for changes in. The baking soda and vinegar reaction is fairly short lived and depending on how many kids you have playing and how big they like to play you might run out of supplies pretty quickly. Vinegar or Acetic Acid has the chemical formula CH3COOH.
The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions. As you may be aware baking soda and vinegar are often used together in school science projects to create a bubbling fizzing reaction. This post contains affiliate links Exploring Chemical Reactions with Baking.
Balanced Chemical Equation for Baking Soda and Vinegar Reaction One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate one mole of water and one mole of carbon dioxide. Baking soda is a base also known as Sodium Bicarbonate and has the chemical formula NaHCO3. Acetic acid present in vinegar will readily react with baking soda sodium bicarbonate to form sodium acetate with the effervescence of carbon dioxide.
NaHCO 3 HC 2 H 3 O 2 NaC 2 H 3 O 2 H 2 O CO 2. Watch Queue Queue When baking soda sodium bicarbonate is combined with any type of vinegar dilute acetic acid or citrus juice citric acid carbon dioxide gas and sodium acetate form and produce a pretty spectacular bubbling reaction. This reaction creates sodium acetate water and carbon dioxide gas.
Not all chemical reactions need heat energy to make them happen. It is always fun to observe. When carbon dioxide is released we see it as a bubbling effect foam and fizz.
When bicarbonate of soda and vinegar are mixed the chemical reaction produces a gas. The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions. The baking soda and vinegar reaction is actually two separate reactions.
The reaction between baking soda and vinegar actually occurs in two steps but the overall process can be summarized by the following word equation. Baking soda vinegar carbon dioxide water sodium acetate NaHCO. This activity explores the popular baking soda and vinegar reaction which is a simple acid-base chemical reaction.
During this reaction the products are sodium acetate C2H3NaO2. Baking soda and vinegar chemical reaction Chemical reactions occur when 2 molecules interact to form new compounds or molecules. Baking soda is bicarbonate NaHCO3 and vinegar is acetic acid HCH3COO.
When the baking soda meets the vinegar there is a chemical reaction as carbon dioxide gas is created and fills the balloon causing it to inflate. The reaction proceeds in two steps. Fun investigation into how adding more vinegar changes the baking soda and vinegar reaction.
These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below. These two ingredients are so widely used in schools because they are a great example of a chemical reaction without the fear of children getting hurt in the process. The reaction proceeds in two steps.
We decided to add food coloring to the ingredient list to prompt even more discoveries. Created by LABScI at Stanford 4. Follow our Science for Kids Pinterest board.
CH3COOH NaHCO3 CH3COONa CO2 H2O. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Kids always love experimenting with baking soda and vinegar.
This clip is from. One of the products this reaction creates is carbon dioxide which makes the bubbles. The chemical equation for the overall reaction is.
When baking soda and vinegar mix together a chemical reaction is triggered.
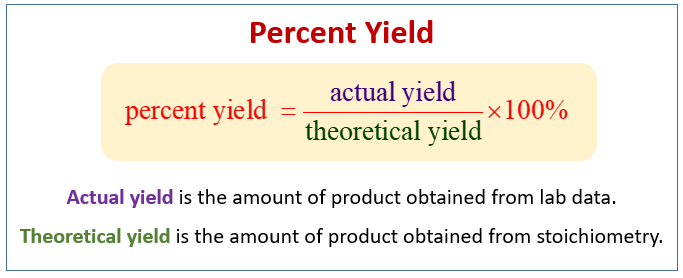
Calculating percentage yield The percentage yield is calculated using this equation. The reaction yield absolute yield of a chemical reaction is the amount of pure and dry product yielded in a reaction.
 Stoichiometry And Percent Yield Examples Solutions Worksheets Videos Games Activities
Stoichiometry And Percent Yield Examples Solutions Worksheets Videos Games Activities
Normally in order to measure the efficiency of a chemical reaction in organic synthesis the relative or percentage yield is calculated.
Percent yield of a reaction. Calculating the percent yield helps you to know the effectiveness of your performed reaction. CaCO₃ CaO CO₂. Percentage yield frac actual yield theoretical yield times 100 The percentage yield can vary.
Eg volume if the product is a gas. Assume that none of the substances were wasted during the process and no by-products are produced then your percent yield is 100 percent which is almost impossible to achieve. The extent to which a reactions theoretical yield is achieved is commonly expressed as its percent yield.
So what if the sodium hydroxide is not in excess at least we dont know if it is. The equation for percent yield is. Name six methods of separating materials.
Actual yield is the amount of product obtained from a chemical reaction. For the reaction NH4NO3 s N2O g 2H2O l you decompose 60 g of NH4NO3 and get 11 g of N2O. What is the percent yield of the following reaction if 60 grams of CaCO3 is heated to give 15 grams of CaO.
It is not always possible to achieve 100 yield in a chemical reaction. Sometimes its possible for your percent yield to be above 100 percent. Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis processes.
Chemistry Stoichiometry Percent Yield. The amount of product actually produced is called the actual yield. What is the percent yield.
Explain why percentage yield may be less than 100 Example. A percent yield of 90 means the reaction was 90 efficient and 10 of the materials were wasted they failed to react or their products were not captured. To express the efficiency of a reaction you can calculate the percent yield using this formula.
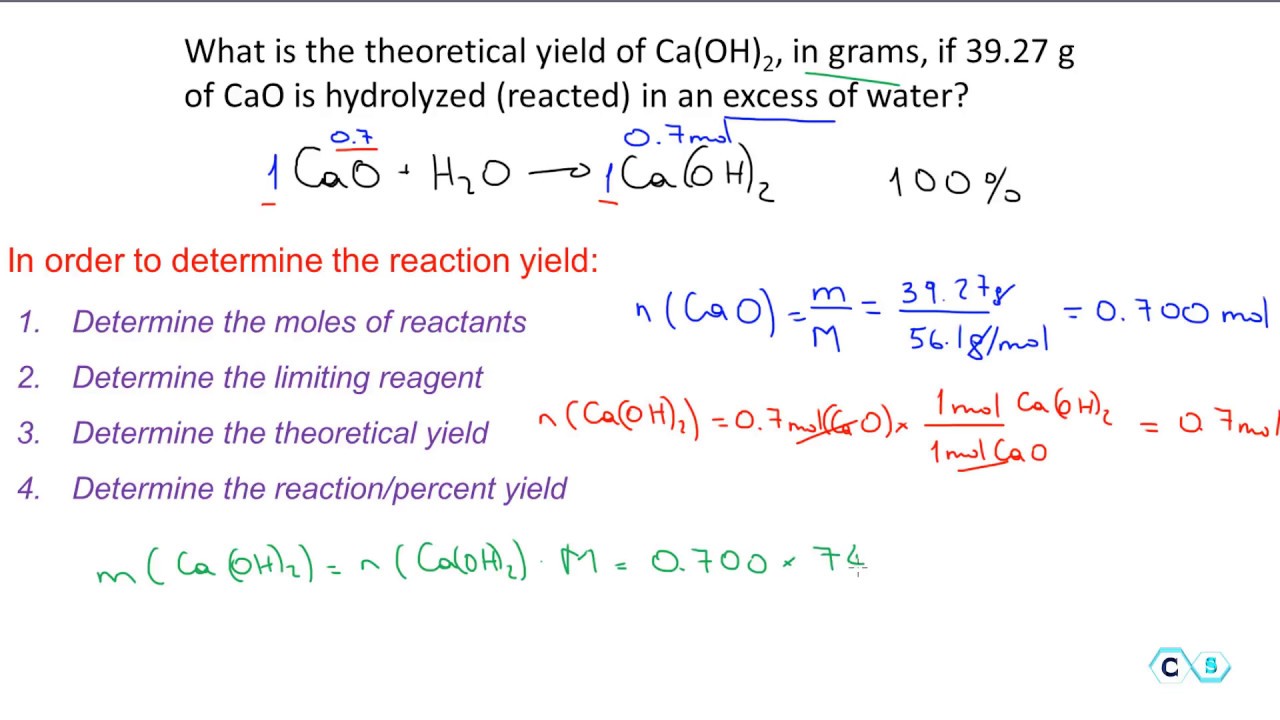
You want to measure how much water is produced when 120 g of glucose C6H 12O6 is burned with enough oxygen. Theoretical yield is the amount of product obtained from the stoichiometric or balanced equation using the limiting reactant to determine product. How to calculate the percent yield of a chemical reaction.
The combined mass of both reagents and containers is 25598 g. Yield actual yieldtheoretical yield x 100. Percent yield actual yield theoretical yield 100 Actual and theoretical yields may be expressed as masses or molar amounts or any other appropriate property.
1291 Percent Yield Actual Yield Theoretical Yield 100. Percent yield actual yieldtheoretical yield x 100. 1 Answer Ernest Z.
The formula for percent yield is the experimental yield divided by the calculated theoretical yield. The theoretical yield is what you calculate when you do a calculation on paper or before you do a reaction in a lab. The extent to which a reactions theoretical yield is achieved is commonly expressed as its percent yield.
The actual yield will always be less than the theoretical yield because no chemical reaction ever reaches 100 percent completion. Percent yield is a measure of how well the reaction proceeded to completion. Sep 18 2014 The percent yield is 45.
Before calculating the yield of a reaction necessary when preparing the laboratory notebook it is crucial to know the stoichiometry of a reaction adjusting the chemical reaction so that there are the same numbers and types of atoms. Yield actual yield theoretical yield 100 So lets say you want to do an experiment in the lab. Some of the product may be lost when it is separated from the reaction mixture.
Percent yield actual yield theoretical yield 100 percent yield actual yield theoretical yield 100. Its going to be 04 moles over 05 moles times 100 and we have 80. In chemistry yield also referred to as reaction yield is a measure of the quantity of moles of a product formed in relation to the reactant consumed obtained in a chemical reaction usually expressed as a percentage.
How to calculate the percentage yield for a reaction. Assume that the sulfuric acid is unlimited. The calculated or expected amount of product is called the theoretical yield.
The percent yield is the ratio of the actual yield to the theoretical yield expressed as a percentage. So the yield of this reaction is 80. When you divide actual yield by theoretical yield you get a decimal percentage known as the percent yield of a reaction.
In chemical reaction engineering yield conversion and selectivity are terms used to describe ratios of how much of a reactant was consumed how much d. Calculate the mass of magnesium sulfate that could be produced from 48 g of magnesium. The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory.
Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. The percent yield of this reaction is going to be the actual yield divided by the theoretical yield multiplied by 100. Consider a 352-g sample of CaCO 3 9987 pure in a flask and a 1000 mL sample of vinegar 5 acidity in a graduated cylinder.
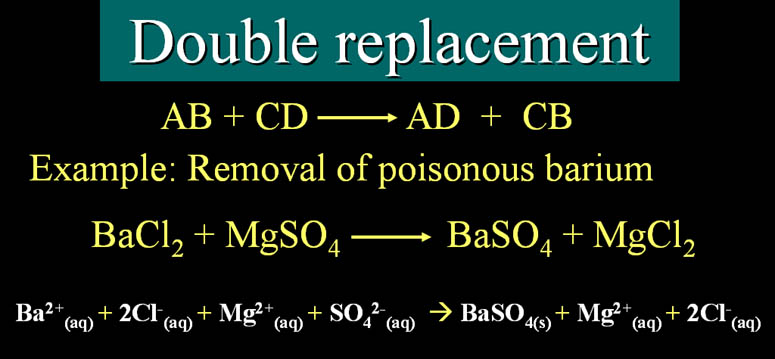
5 Main Patterns of Reactions Synthesis Decomposition Single Displacement Double Displacement Combustion Synthesis To build or put together Two separate things joining to form. Double displacement Reaction - definition.
 Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com
Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com
Double replacement reaction - a chemical reaction.

Double displacement reaction definition. Examples of Displacement Reactions Examples of single displacement reaction Reaction between zinc and copper sulphate. Predicting and balancing neutralization and precipitation reactions. This reaction indeed looks like a double replacement reaction.
A chemical reaction between two compounds in which the first and second parts of one reactant are united respectively with the second and first parts. Double Displacement Reaction Definition and Examples A double displacement reaction is a type of reaction where two reactants exchange ions to form two new compounds. This reaction is represented by the general scheme.
A double displacement reaction also known as a double replacement reaction or metathesis is a type of chemical reaction where two compounds react and the positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. The reaction given below AgNO 3 NaCl AgCl NaNO 3. For example N a2.
During this reaction the cations and anions of two different compounds switch places forming two entirely different compounds. Double displacement reactions may be defined as the chemical reactions in which one component each of both the reacting molecules is exchanged to form the products. The process in which the positive and negative ions of the reactant involved in a chemical reaction exchange places forming new compounds is called a double.
Double replacement reaction synonyms double replacement reaction pronunciation double replacement reaction translation English dictionary definition of double replacement reaction. A double displacement reaction is a type of chemical reaction where two compounds react and positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products.
AB CD AD CB An example is the reaction between silver nitrate and sodium chloride to form silver chloride and sodium nitrate. Double decomposition double decomposition reaction metathesis a chemical reaction between two compounds in which parts of each are interchanged to form two new. Usually a double displacement reaction results in precipitate formation.
In double displacement reactions two chemical species are displaced. I was taught that double displacement reactions occur when reactants are aqueous. A double displacement reaction is a chemical reaction that involves the exchange of two ionic species between two different molecules.
Double displacement synonyms double displacement pronunciation double displacement translation English dictionary definition of double displacement. Example of double displacement reaction Reaction between silver nitrate and sodium chloride is an example of double displacement reaction. Types of Reactions Synthesis Decomposition Single and Double Displacement Displacement Combustion on SNC2D7 - Grade 10 Science Exam Prep.
However I came across this reaction ceZnOs H2S g - ZnSs H2O g This is the reaction that is used to remove hydrogen sulfide that is naturally found in methane gas. In displacement reactions one chemical species is displaced. Define double replacement reaction.
The chemical bonds between the reactants may be either covalent or ionic. English dictionary definition of double replacement reaction. Double displacement reactions typically result in the formation of a product that is a precipitate.
This ScienceStruck post explains the concept of double displacement reaction in chemistry along with a few examples. A salt metathesis reaction sometimes called a double replacement reaction double displacement reaction is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. Double displacement reactions are reactions where the cations and anions in the reactants switch partners to form products.
1 n a chemical reaction between two compounds where the positive ion of one compound is exchanged with the positive ion of another compound Type of. Definition and examples of double replacement reactions. Double displacement reactions are those in which the reactant compounds exchange positive ions with each other to form new compounds.
A double displacement reaction also known as a double replacement reaction or metathesis is a type of chemical reaction where two compounds react and the positive ions cation and the negative.
The reaction takes place between different metals or halogens. A few real life examples include the process of rusting where zinc undergoes a single replacement with steel and the Statue of Liberty where there was a single replacement reaction between the outer layer of copper and an inner layer of iron.
 Single Replacement Reactions And Net Ionic Equations Youtube
Single Replacement Reactions And Net Ionic Equations Youtube
2Fe2O3 3C 4Fe 3CO2.
Single replacement reaction examples. CHEMISTRY SINGLE REPLACEMENT REACTION WORKSHEET Using the Activity Series Table complete the following reactions by writing the products that are formed. Cl 2 NaBr --- NaCl Br 2 Br 2 KI --- KBr I 2. An example of a single replacement reaction occurs when potassium K reacts with water H 2 O.
2LiBr aq F 2 g Br 2 g 2LiFaq 16. This video contains plent. Single-Displacement Reaction Examples The reaction between zinc metal and hydrochloric acid to produce zinc chloride and hydrogen gas is an example of a single-displacement reaction.
In the same way for a single-displacement reaction an element can only be replaced if the element taking its place is more reactive. If No single replacement reaction occurs write NR to the right of the arrow. Look for signs of a reaction.
Single Replacement Reaction Examples There are two different scenarios for single replacement reactions. The only examples the ChemTeam knows about involve halogens so here are two examples. A colorless solid compound named potassium hydroxide KOH forms and hydrogen gas H 2 is set free.
Al H 2 SO 4 4. In one form of the reaction one cation replaces the other. In single replacement one reactant is always an element.
Chemical reactions often involve color changes temperature changes gas production or precipitant formation. A common single replacement reaction occurs when zinc and copper move from aquous solutions to solids in batteries after electrons to create power. 1172 A BC AC B In this general reaction element A is a metal and replaces element B also a metal in the compound.
A single-displacement reaction also known as a single-replacement reaction is a type of chemical reaction in which an element reacts with a compound and takes the place of another element within. It can be represented generically as. In the extraction of iron from its ore ferric oxide is heated with carbon.
Real life examples of single replacement reactions include the exterior of the Statue of Liberty and processes in the steel industry. The equation for the reaction is. A single-displacement reaction also known as single replacement reaction or exchange reaction is a chemical reaction in which one element is replaced by another in a compound.
Zinc gives up two electrons and copper gains electrons in single replacement reactions with electricity. A single replacement reaction occurs when an element reacts with a compound displacing another element in that compound according to the University of Memphis. Li H 2.
Simple examples of everyday reactions include digestion combustion and cooking. The general form of a single-replacement also called single-displacement reaction is. Chemical reactions are common in daily life but you may not recognize them.
In our surroundings we encounter some examples of single replacement reaction. Zn CuCl2 ZnCl2 Cu. Two examples are also sho.
In the other form of the reaction one anion replaces the other. This chemistry video tutorial explains how to write the products of a single replacement reaction and find the net ionic equation. Where and are different metals or any element that forms cation like hydrogen and is an anion.
Carbon displaces iron at very high temperature and then elemental iron is formed. Inna BigunShutterstock To understand this better lets consider the following examples. Ag KNO 3 2.
Predict the products and write the correct balanced equation for the single replacement reaction between lithium bromide and fluorine. A single replacement reaction sometimes called a single displacement reaction is a reaction in which one element is substituted for another element in a compound. The starting materials are always pure elements such as a pure zinc metal or hydrogen gas plus an aqueous compound.
The other reactant will be a compound. And are halogens and is a cation. For single-displacement reactions a metal replaces a metal.
Zns 2 HClaq ZnCl 2 aq H 2 g. 2K 2H 2 O 2KOH H 2. A single-replacement reaction is a reaction in which one element replaces a similar element in a compound.
A diagrammatic representation of Single Replacement Reaction Photo Credit. Cl 2 KI 5. A single replacement reaction occurs when one substance replaces another in a chemical reaction.
Describes the basics of single replacement reactions how to identify them predict the product and balance the chemical equation. The compound FBr is not an ionic compound and is not likely to form in a single replacement reaction. Be sure to Balance each equation.
It does not matter if the element is written first or second on the reactant side. Zn AgNO 3 3. Only a single compound undergoes replacement in this reaction thus the name single replacement.