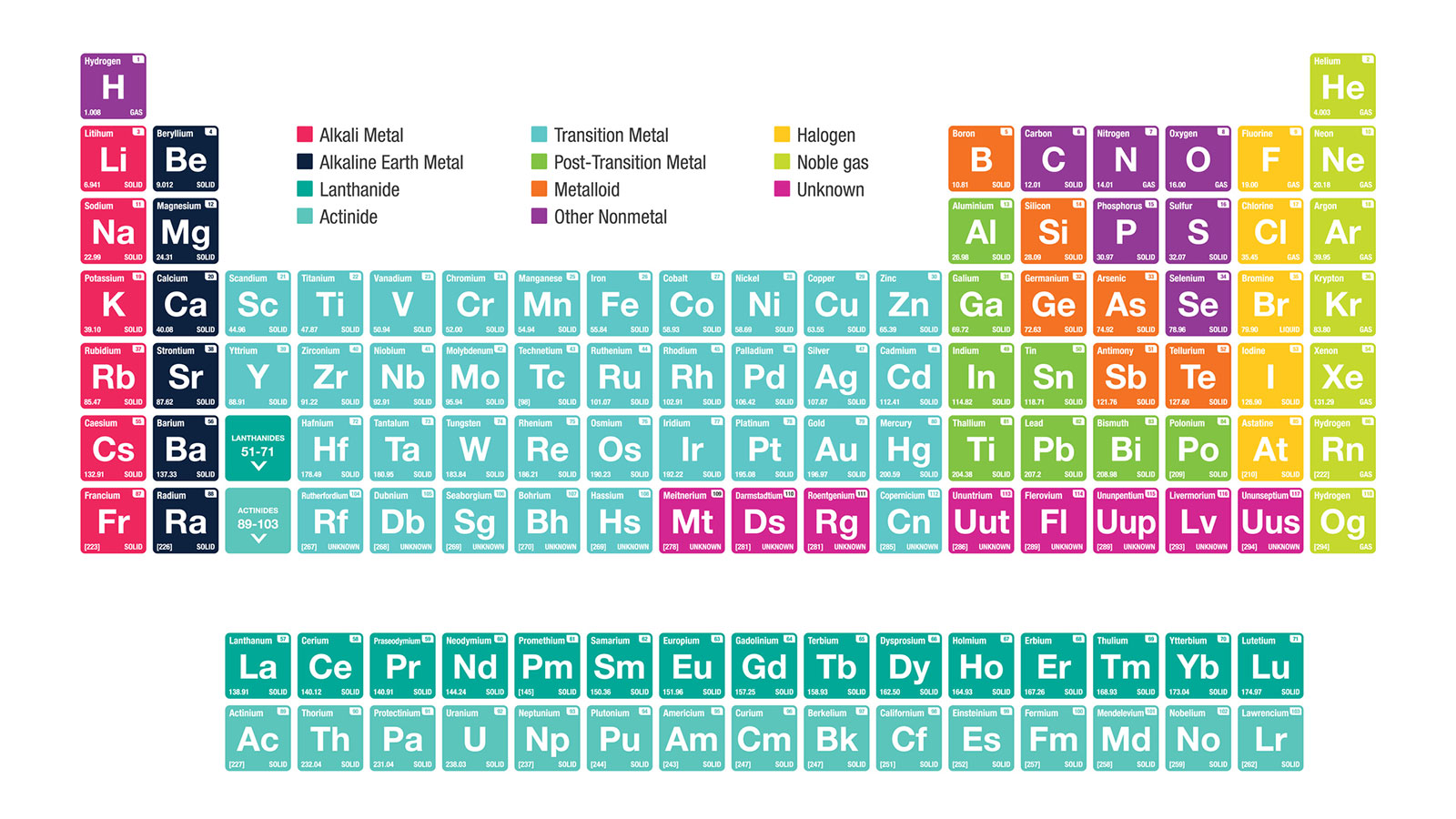
The alkali metals are lithium sodium potassium rubidium cesium and francium. In the periodic table the alkali metals are a group or column containing the chemical elements such as lithium Li sodium Na rubidium Rb potassium K francium Fr and Caesium Cs.
 Alkali Metals Facts About The Elements On The First Column Of The Periodic Table Howstuffworks
Alkali Metals Facts About The Elements On The First Column Of The Periodic Table Howstuffworks
This group includes the elements lithium sodium potassium rubidium caesium and franciumEach of these elements has just one valence electron which means that they form.

Alkali metals periodic table. The only element in the first column that is not usually considered an alkali metal is hydrogen. Alkali metals are found in which group of the periodic table. The alkali metals have the high thermal and electrical conductivity lustrous ductile and malleable that are characteristic of metals.
The alkali metals are lithium sodium potassium rubidium cesium and francium. This video below explains more about alkali metals or group 1 metals on the periodic table. This makes it easier for an atom to lose an electron from its outer shell.
Preview this quiz on Quizizz. Lithium Li sodium Na potassium K rubidium Rb caesium Cs francium Fr Properties of alkali metals. This is because the outer shell gets further away from the positive attraction of the nucleus.
The Periodic Table - the Alkali Metals. The alkali metals are so called because reaction with water forms alkalies ie strong bases capable of neutralizing acids. This common electron setup.
The metals whose oxides make up the alkaline earths then came to be known as the alkaline-earth metals and have been classified in Group 2 IIa of the periodic table ever since Russian chemist Dmitry Mendeleyev proposed his first table in 1869. Kids Learning Tube Learn about the Periodic Table of elements and the group alkali metals with this fun educational music video for children and parents. 19 Number of Neutrons.
Alkali metals are found in which group of the periodic table. When looking for families the first one you will find is the alkali metal family of elements. You should remember that there is a separate group called the alkaline earth metals in Group Two.
This group lies in the s-block of the periodic table as all alkali metals have their peripheral electron in an s-orbital. Francium is so radioactive and short-lived that nobody has ever seen a lump of it. The reaction is very vigorous and can sometimes result in explosions.
390 Number of ProtonsElectrons. 10th - 11th grade. Play this game to review Periodic Table.
Sir Humphrey Davy Uses. And so if this video will be talking about Group One which are the alkali metals and so alkaline metals are basically medals in Group one excluding hydrogen. BamlouGetty Images There are alkali metals all around you right now.
They all go on earth metals which are group too. They are all in the first column of the periodic table. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right.
General properties of alkali metals. They are also known as the alkaline metals. Alkali metals become more reactive as you go down the group in the periodic table.
This page introduces the Alkali Metals in Group 1 of the Periodic Table. The alkali metals are the elements located in Group IA of the periodic table. Alkali metal any of the six chemical elements that make up Group 1 Ia of the periodic table namely lithium Li sodium Na potassium K rubidium Rb cesium Cs and francium Fr.
And we also have our transition metals which is in the D block of the periodic table as well as the Atlanta nods and the active. It is the first group of s-block Despite the presence of hydrogen at the top of the group 1A It is not one of the alkali metals but it is one of the nonmetals because it has a small atomic size and it is a gas. Lets go to the left side of the periodic table.
Alkali metals make up six different elements found in the first column of the periodic table. The elements in group one of the periodic table with the exception of hydrogen - see below are known as the alkali metals because they form alkaline solutions when they react with water. Hydrogen and the alkali metals make up the group 1 elements of the periodic table.
We can however predict what its properties might. Alkali metals group is located on the maximum left side of the modern periodic table. The alkali metals are a group of elements in the periodic table.
Alkali and Alkaline Earth Metals. Sodium is found in table salt lithium in your phone battery and potassium in your bananas. As with the alkali metals of Group 1 Ia the atoms of the alkaline-earth metals easily lose.
The alkali metals are on the left column of the periodic table highlighted in hot pink. The alkali metals are among the most electropositive elements on the periodic table and thus tend to bond ionically to the most electronegative elements on the periodic table the halogens fluorine chlorine bromine iodine and astatine forming salts known as the alkali metal halides.