6 bonding regions 0 lone pairs. Since only those electrons in the valence or outer shell of an atom participate in bonding lone pairs are studied in chemistry to account for the different shapes of molecules with the same number of bonds.
 1 6 Lewis Structures Chemistry Libretexts
1 6 Lewis Structures Chemistry Libretexts
A lone pair references a pair of electrons in the valence shell of an atom which are not bonded to another atom or molecule.
What is a lone pair. I 3-6 Regions of High Electron Density Octahedral Arrangement. A lone pair in chemistry refers to a pair of valence electrons that in a covalent bond are not exchanged with another atom and is often called an unshared pair or non-bonding pair. The lone pair concept is important to valence shell electron pair repulsion VSEPR theory as it helps to explain the geometry of molecules.
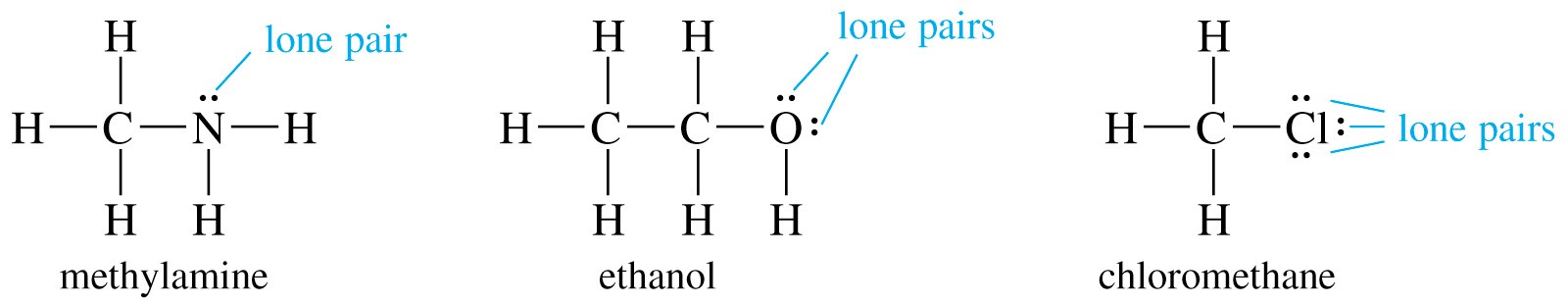
One way to identify a lone pair is to draw a Lewis structure. Give the number of lone pairs around the central atom and the geometry of the ion ClO 3. ICl3 has two lone pairs of electrons one above the I one below.
N chem a pair of valency electrons of opposite spin that are not shared between the atoms in a molecule and are responsible for the formation of coordinate. Therefore a lone pair is also called a non-bonding electron pair. The number of lone pairs on chlorine atom is ClO- ClO-2 ClO-3 ClO-4 ions are 1.
Look it up now. The number of lone pair electrons added to the number of bonding electrons equals the number of valence electrons of an atom. IF 6 5 bonding regions 1 lone pair.
These bonds are not especially strong but because they orient the water molecules into a specific configuration. Distribution of regions of high electron density. Lone pair synonyms lone pair pronunciation lone pair translation English dictionary definition of lone pair.
Coordination number refers to the number of electron pairs that surround a given atom often referred to as the central atom. In the outermost electron shell of atoms lone pairs are found. The electron-pair geometry for eqdisplaystyle rm Be eq in eqdisplaystyle rm BeI_2 eq is linear.
The geometries of molecules with lone pairs will differ from those without lone pairs because the lone pair looks like empty space in a molecule. Although electrons in the innermost shells are also coupled and do not participate in the bonding they are not considered as lone pairs. Get answers by asking now.
The electrons of the lone pair belong to the same atom. A lone pair is an electron pair in the outermost shell of an atom that is not shared or bonded to another atom. There are lone pairs around the central atom so the geometry of seh2 is.
A lone pair is a group of two electrons that are not used in any bonds between atoms. It is also called a non-bonding pair. As an example the two oxygens of an ester group possess localized and delocalized lone pairs.
Each E represents a lone pair of electrons on the central atom. The electron pairs around a central atom are represented by a formula AX n E m where A represents the central atom and always has an implied subscript one. 2 bonding regions 3 lone pairs.
Number of nitrogen atoms in the N 2 O molecule 2. They are usually high in energy. In other words a lone pair is a non bonding pair.
A lone pair consists of 2 electrons in the same orbital from the same atom which are not involved in bonding. In a similar way the same element in one molecule can have localized and delocalized lone pairs of electrons. They are always in the last shell of an atom the valence shell.
Together with the electrons used in bonding they make up the total number of valence electrons. 3 bonding regions 2 lone pairs. There are zero lone pairs around the central atom so the geometry of eq.
The lone pair consists of two electrons that normally belong to the same atom. Lone pair is a pair of electrons that are not in a bond. They can be identified by using a Lewis structure.
The number of lone pair electrons added to the number of bonding electrons equals the number of valence electrons of an atom. Each X represents a ligand an atom bonded to A. Lone pairs are found in the outermost electron shell of atoms.
Lone pair is generally not involved in bonding formation so can also say that lone pair is known as no-bonding electron pair. The term lone pair can describe as the electron pairs that are usually not bonded. By using a Lewis structure they can be defined.
The total number of X and E is known as the steric number. The red electrons on the oxygen can participate in resonance stabilization because of the possibility of moving up the pi bond electrons. A dash or line is sometimes used to indicate a shared pair of electrons.
The water molecule Known as the lone-pair orbitals these are the keys to waters peculiar behaviour in that they attract the hydrogen nuclei of adjacent water molecules to form what are called hydrogen bonds. In chemistry a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pair.