This notation uses the symbol of the previous rows noble gas in brackets to represent the part of the electron configuration that is identical to. Chemical elements listed by atomic mass The elements of the periodic table sorted by atomic mass.
 Color Periodic Table For Kids 2 Decimal Point Atomic Masses To Make Calculations Easier To Ma Periodic Table Printable Science Notes Chemistry Periodic Table
Color Periodic Table For Kids 2 Decimal Point Atomic Masses To Make Calculations Easier To Ma Periodic Table Printable Science Notes Chemistry Periodic Table
Visit BYJUS to learn more about it.
%252C_black_and_white.png?revision=2)
Periodic table with mass. Every element has its own mass and which is not the same therefore it is difficult to find out the mass value of an element and even a slight change can affect the whole experiment. Here we present the HD images of the periodic table with the atomic mass of the elements on our website. The following article will help you to gain more information about the same.
Periodic Table with Mass. The periodic table provides us with a comprehensive view of the various elements. Each atomic mass is.
Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. A good periodic table is a necessary part of every chemists or future chemists reference materials. Periodic Table With Names And Atomic Mass.
This color periodic table contains the accepted standard atomic weights atomic masses of each element as accepted by the IUPACThe IUPAC doesnt update these values annually so these are the most recent values for 2019. Metals reside on the left side of the table while non-metals reside on the right. Definition mass chemical names.
1 H Hydrogen 1008 1. Periodic Table With Mass Periodic Table Atomic Mass Chart Online Periodic Table Atomic Mass Image Download Periodic Table Atomic Mass Chart Save Periodic Table Atomic Mass Image Print Periodic Table Atomic Mass Format Periodic Table Atomic Mass Image Printable Periodic Table Atomic Mass. Interactive periodic table with up-to-date element property data collected from authoritative sources.
Interactive periodic table showing names electrons and oxidation states. Black and White PDF Periodic Table for Kids The 118 element tiles have a colored border that indicates the element group or else are black and white. Download and print a Periodic Table with Names Atomic mass Charges and other properties.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. Periodic Table of Elements - The periodic table is a very useful listing of all 118 elements by symbol atomic number and atomic mass and molecular mass. Periodic Table With No Names This is the table to use if youre learning the element names or just want a cleaner table a periodic table with symbols and numbers.
Mass value plays an important role when working in laboratories because it can be a matter of success experiment or failure. The periodic table was arranged by atomic mass and this nearly always gives the same order as the atomic number. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game.
This is a rundown of synthetic components arranged by nuclear mass or most stable isotope and shading coded by kind of componentEvery components nuclear number name component image and gathering and period numbers on the intermittent table are given. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Click on any elements name for further information on chemical properties environmental data or health effects.
The electron configurations are written in the noble gas notation. IOS app is also available. An easy to use guide to the elements of the periodic table including their history discovery physical and chemical characteristics their uses significance and much more.
Elements with similar chemical properties are called groups. This color periodic table wallpaper contains each elements atomic number atomic mass symbol name and electron configuration. Options for hiding the symbol or name of the elements provide a handy learning aid for memorizing the periodic table.
Use this periodic table for calculating molar mass for any chemical formula. Along with atomic mass and atomic number values this table helps us to understand the various properties abbreviations and names of all the elements present in nature. Mendeleev had seen that they needed to be swapped around but it was Moseley that finally determined why.
Periodic table with atomic mass the atomic mass of any chemical event is the important property which is taken into consideration while conducting the research or experiment on any chemical event therefore you must have the proper information about the atomic mass of the concerned element. This list contains the 118 elements of chemistry. After looking around for a useful printable periodic table I found that most were pretty basic and included only a few properties.
Printable periodic table of the elements. Visualize trends 3D orbitals isotopes and mix compounds. However there were some exceptions like iodine and tellurium see above which didnt work.
The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties. 2019 Periodic Table of the Elements.
Atomic Mass Versus Mass Number The mass number is the sum of the number of protons and neutrons in an atom. Are all the Subatomic Particles considered while measuring Atomic.
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png) Difference Between Atomic Weight And Atomic Mass
Difference Between Atomic Weight And Atomic Mass
Although the SI unit of mass is kilogram the atomic mass is often expressed in the non-SI unit dalton where 1 dalton is defined as 112 of the mass of a single carbon-12 atom at rest.

What is atomic mass. A single atom has a set number of protons and neutrons so the mass is unequivocal wont change and is the sum of the number of protons and neutrons in the atom. Atomic mass is the sum of the masses of the protons neutrons and electrons in an atom or the average mass in a group of atoms. Electrons contribute so little mass that they arent counted.
Though technically incorrect the term is also often used to refer to the average atomic mass of all of the isotopes of one element. Atomic Mass is an extremely. M a is the mass of a single atom of a chemical element.
Atomic mass m a is the mass of an atom. Da or u is a unit of mass widely used in physics and chemistry. It includes the masses of the 3 subatomic particles that make up an atom.
Atomic Mass or Weight Definition Atomic mass which is also known as atomic weight is the average mass of atoms of an element calculated using the relative abundance of isotopes in a naturally occurring element. Atomic mass of an element may be defined as the average relative mass of an atom of the element as compared with mass of an atom of carbon C-12 isotope taken as 12 amu. Thus the numeric value of the atomic mass when expressed in daltons has nearly the same value as the.
The atomic mass is carried by the atomic nucleus which occupies only about 10 -12 of the total volume of the atom or less but it contains all the positive charge and at least 9995 of the total mass of the atom. Atomic mass - chemistry the mass of an atom of a chemical element expressed in atomic mass units atomic weight relative atomic mass mass - the property of a body that causes it to have weight in a gravitational field. But how is it exactly Measured.
The atomic mass constant denoted m u is defined identically giving m u m12 C12 1 Da. The mass number is a count of the total number of protons and neutrons in an atoms nucleus. However electrons have so much less mass than protons and neutrons that they dont factor into the calculation.
This second definition is actually the relative atomic. In chemistry an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon -12. It is expressed as a multiple of one-twelfth the mass of the carbon-12 atom 1992646547 10 23 gram which is assigned an atomic mass of 12 units.
It is a weighed average of the different isotopes of an element. The atomic mass is the mass of an atom. Atomic mass is the weighted average mass of an atom of an element based on the relative natural abundance of that elements isotopes.
The dalton or unified atomic mass unit symbols. Atomic weight is measured in atomic mass units amu also called daltons. So the atomic mass is the sum of the masses of protons and neutrons.
However because each atom has a very small mass this is not very helpful. Atomic mass can be expressed in grams. Atomic weight ratio of the average mass of a chemical elements atoms to some standard.
This unit is commonly used in physics and chemistry to. Atomic mass is the sum of all the protons neutrons and electrons in a single atom or molecule. Atomic Mass is an extremely important concept in Chemistry.
It is a unit of mass used to express atomic masses and molecular masses. It has been found experimentally that the mass of one carbon-12 atom is 199265 10 26 kg. The mass of an isotope of an element measured in units formerly based on the mass of one hydrogen atom taken as a unit or on 116 00625 the mass of one oxygen atom but after 1961 based on 112 00833 the mass of the carbon-12 atom.
The protons and neutrons of the nucleus account for nearly all of the total mass of atoms with the electrons and nuclear binding energy making minor contributions. However the mass of an electron is so small it is considered negligible and not included in the calculation. An atomic mass symbol.
Protons neutrons and electrons. It is defined as 112 of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. Atomic mass the quantity of matter contained in an atom of an element.
In this scale 1 atomic mass unit amu corresponds to 1660539040 10 24 gram. Since 1961 the standard unit of atomic mass has been one-twelfth the mass of an atom of the isotope carbon-12. Therefore 1 u is defined as of the mass of a carbon-12 atom.
Atomic mass indicates the size of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element.
The mass can neither be created nor destroyed. The law of conservation of matter or principle of matter conservation states that the mass of an object or collection of objects never changes over time no matter how the constituent parts rearrange themselves.
In other words the mass of an object or collection of objects never changes no matter how the parts are rearranged.

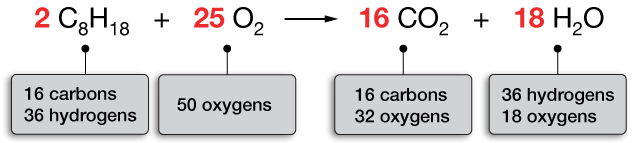
What is the law of conservation of mass. The Law of Conservation of Matter Conservation of Mass The law of conservation of matter or principle of matter conservation states that the mass of an object or collection of objects never changes over time no matter how the constituent parts rearrange themselves. In physics and chemistry the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy the mass of the system must remain constant over time as the systems mass cannot change so quantity can neither be added nor be removed. The law of conservation of mass states that no atoms are lost or made in a chemical reaction.
According to the law of conservation of mass the mass of the products in a chemical reaction must equal the mass of the reactants. The law implies that mass can neither be created nor destroyed although it may be rearranged in space or the entities associated with it. Instead the atoms join together in different ways to form products.
Mass has been viewed in physics in two compatible ways. Sir Antoine Lavoisier promoted this idea. Conservation of mass definition is - a principle in classical physics.
In chemistry the law of conservation of mass states that the mass of the products the chemical substances created by a chemical reaction will always equal the mass of the reactants the substances that make the chemical reaction. It can change forms but is conserved. The law of conservation of mass is a fundamental principle of physics.
This best demonstrates the law that states matter cannot be created or destroyed. Law of conservation of mass No atoms are created or destroyed in a chemical reaction. Instead it is transformed into other forms.
Instead they just join together in a different way than they were before the reaction and form products. In this process the mass of the paper is not actually destroyed. The law of conservation of mass states that mass in a closed system--where nothing is added or taken away--will stay the same.
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to this law matter can be neither created nor destroyed. The American Heritage Science Dictionary Copyright 2011.
The mass can neither be created nor destroyed. Conservation law in physics several principles that state that certain physical properties ie measurable quantities do not change in the course of time within an isolated physical system. The principle does not hold under Special Relativity since mass and energy can be converted into one another.
In classical physics laws of this type govern energy momentum angular momentum mass and electric. The Law of Conservation of Matter Conservation of Mass. A principle of classical physics stating that the total mass of a closed system is unchanged by interaction of its parts.
Conservation of matter constant mass law of Conservation of mass principle that the mass of an object or collection of objects never changes no matter how the constituent parts rearrange themselves. Think of it as being similar to balancing an algebraic equation. The Law of Conservation of Mass is the principle that states that neither physical transformation nor chemical reactions create or destroy mass in an isolated system.
Thus the amount of matter cannot change. Law of Conservation of Mass in Chemistry. Therefore the quantity of mass is conserved over time.
This is why in a balanced symbol. An example of the law of conservation of mass is the combustion of a piece of paper to form ash water vapor and carbon dioxide. The total mass of any isolated material system is neither increased nor diminished by reactions between the parts called also conservation of matter.
The law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction. Law of Conservation of Mass Definition The law of conservation of mass is that in a closed or isolated system matter cannot be created or destroyed. According to this principle the reactants and products in a chemical reaction must have equal masses.
YouTube star Rosanna Pasino bakes scienceinspired treats However Ruben Meerman co-author of the study refutes this and says that this perception violates the Law of Conservation of Mass.
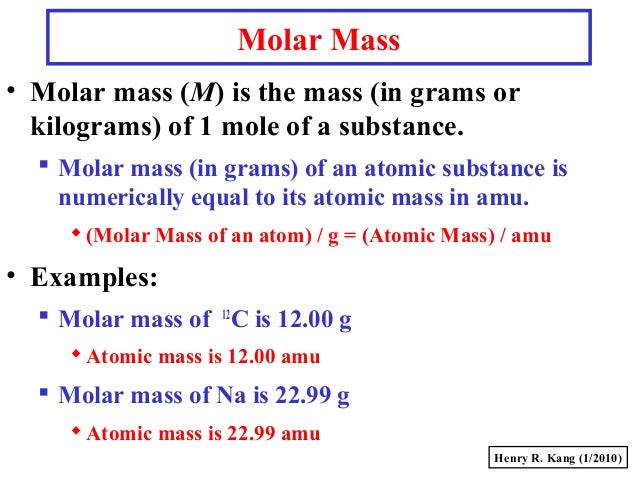
It is the mass of a mole of a substance. What is Atomic Mass.
 How To Calculate Atomic Mass Atoms And Molecules For Kids Teaching Chemistry Atom
How To Calculate Atomic Mass Atoms And Molecules For Kids Teaching Chemistry Atom
The atomic mass of an element is frequently used by chemists to determine the amount of substance required in a chemical reaction.
Atomic mass definition chemistry. The mass number reports the mass of the atoms nucleus in atomic mass units amu. It relates the mass of an element to the number of atoms. The relative atomic mass of an element is a weighted mean mass of the isotopes of an element compared with that of the 12C isotope which has a mass of exactly 12.
The daltonor unified atomic mass unitsymbols. It is defined as 112 of the mass of an unboundneutral atom of carbon-12in its nuclear and electronic ground stateand at rest. Definition of Atomic Mass Atoms are the basic units of matter.
The atomic mass is the sum of the mass of protons neutrons and electrons. Atomic Mass Unit AMU The periodic table of elements contains every atom known to mankind. Daor u is a unitof masswidely used in physics and chemistry.
The mass of an atom or a molecule is referred to as the atomic mass. Each unique atom has a unique atomic number and atomic mass. One atomic mass unit is equal to one-twelfth of the mass of an atom of carbon 12 isotope.
The number of protons in an atom of an element is called the atomic number Z. The atomic mass is used to find the average mass of elements and molecules and to solve stoichiometry problems. Medical Definition of atomic mass unit.
The mass number A of an element is the total number of protons and neutrons in an atom of that element. The symbol used for it is M. It is denoted by ma.
Atomic mass - chemistry the mass of an atom of a chemical element expressed in atomic mass units atomic weight relative atomic mass mass - the property of a body that causes it to have weight in a gravitational field. The sum of the mass number and the atomic number for an atom A-Z corresponds to the total number of subatomic particles present in the atom. Updated June 30 2019 In chemistry an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon -12.
Atomic mass definition the mass of an isotope of an element measured in units formerly based on the mass of one hydrogen atom taken as a unit or on 116 00625 the mass of one oxygen atom but after 1961 based on 112 00833 the mass of the carbon-12 atom. Relative atomic masses do not. Mass is a basic physical property of matter.
It has a unit of the unified mass unit u or the atomic mass unit amu. Atomic mass indicates the size of an atom. G mol 1 is the standard unit for the molar mass.
The atomic mass constant denoted muis defined identically giving mu m12C12 1 Da. The atomic number is the number of protons. It is a unit of mass used to express atomic masses and molecular masses.
Atomic mass is a fundamental concept in chemistry. They are the smallest components of a chemical element which is a substance that cannot be broken down into simpler material. Atomic Mass or Weight Definition Atomic mass which is also known as atomic weight is the average mass of atoms of an element calculated using the relative abundance of isotopes in a naturally occurring element.
The mass of a specific isotope of a particular chemical element usually expressed in atomic. Atomic mass of an element is the number of times an atom of that element is heavier than an atom of carbon taken as 12. The number of protons in an atom of an element is its atomic number.
A unit of mass for expressing masses of atoms molecules or nuclear particles equal to ¹₁₂ the mass of a single atom of the most abundant carbon isotope 12C called also dalton.
Mass number on the other hand is the number of protons in the nucleus of that certain atom or element. It is represented as Da and is the standard unit used to indicate the mass of an atom.
Difference Between Atomic Number And Mass Number Definition Explanation With Examples
The difference is that one is a count of the protons in an elements nucleus while the other is a count of both the protons and neutrons.

What is the difference between mass number and atomic mass. To explain further here is an example. Describe the difference between atomic mass and mass number. Electrons are not counted and can be neglected as their size is very small.
It is a whole number. What is Mass Number. Difference Between Atomic Mass and Atomic Number.
What is the difference between Mass Number and Atomic Mass. Atomic mass vs Atomic number. The atomic mass unit is Dalton.
What is the difference between atomic mass and atomic number. The mass excess of a nucleus is defined as the difference between the atomic mass in atomic mass units u and the mass number of the nucleus A. Atomic mass also atomic weight is the total weight of an element.
It is the average weight of an element. The total number of neutrons and protons of an atom is called atomic mass. Atomic mass does not define the type of element whereas Atomic number defines the type of element.
The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. The main difference between Atomic Mass and Atomic Number is that Atomic Mass is the total number of neutrons and protons in the nucleus of an atom whereas Atomic Number is the total number of protons existing in the nucleus of an atom. Electrons contribute so little mass that they arent counted.
The main difference between atomic number and mass number is that the atomic number indicates the number of protons present in an atom whereas the mass number indicates the sum of the number of protons and the number neutrons present in an atom. The mass number is the sum of the number of protons and neutrons in an atom. Atomic mass is the mass of an atom.
Label the differences in subatomic structure of each. 1Molar mass is the mass of one mole per single element while atomic mass is the mass of an atom at rest or is the number of protons and neutrons. This is a concrete and specific number like 2 or 3 while atomic mass units are not commonly integers.
It is a decimal number. Learn vocabulary terms and more with flashcards games and other study tools. 2Molar mass is measured in grams per mole while atomic mass is unitless 3Atomic mass is measured via mass spectrometry while molar mass is computed via atomic weight.
The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Mass number means the total number of neutrons and protons nucleons in a nucleus of an atom. Mass number is the total number of protons and neutrons in the nucleus.
Draw the 3 atoms showing the subatomic structure of each atom described below and then answer the following questions. Atomic mass is associated with the number of neutrons and protons that are present in a particular nucleus of an element. Key Differences between Atomic Mass and Atomic Number Atomic number is represented by Z whereas Atomic mass is represented by A.
Atomic number is the number of protons in a nucleus of an atom. What is the difference between atomic number and mass number. Definition of Atomic Mass Atomic mass is defined as the number of protons and neutrons contained in an individual unit of a substance.
A single atom has a set number of protons and neutrons so the mass is unequivocal wont change and is the sum of the number of protons and neutrons in the atom. Start studying Difference Between Mass Number and Atomic Mass. One difference between atomic mass and molar mass to take note of is that the latter refers to the weight of a mole of a substance while the latter refers to the weight of the molecules of a substance.
Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. You have been a chosen as a candidate to represent all the women in the whole world. Please be sure to use complete sentences.
Conventionally atomic number is written in the left bottom corner of an element whereas the mass number is written in the left upper corner. Mass number is an integer value whereas the atomic mass often is a decimal value. Atomic number is usually the number of protons present in an elements nucleus.
Atomic mass m a is the mass of an atom.