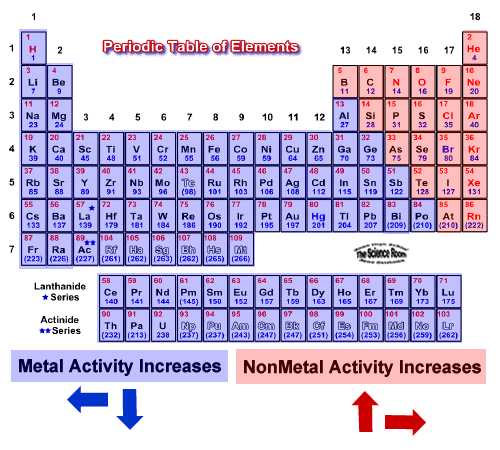
Potassium k is the most reactive metal and platinum pt is the least reactive metal. The most reactive metal on the periodic table is francium.
 What Is The Most Reactive Metal On The Periodic Table Socratic
What Is The Most Reactive Metal On The Periodic Table Socratic
Francium is an alkali metal in group 1IA.
Which is the most reactive metal. The most reactive is at the top the least reactive at the bottom. The activity series of metals with oxygen is given in the image below. Activity series of metals with oxygen When going down the activity series the reactivity of the metal decreases.
The most reactive element is fluorine the first element in the halogen group. It ignites when heated and burns with a brilliant white flame Other metals may be more reactive than magnesium or in between magnesium and platinum. These metals are highly reactive because they all have only one valence electron.
It is the most reactive non-metal. The most reactive metal is Potassium K and the least reactive metal is Gold Au. The alkali metals Li Na K Rb Cs and Fr are the most reactive metals in the periodic table - they all react vigorously or even explosively with cold water resulting in the displacement of hydrogen.
Magnesium is very reactive. Francium however is a laboratory-produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. 0 Pottasium is the most reactive metal and.
The most reactive metal on the periodic table is francium. Gold is the most reactive metal-1. The most reactive metal is francium the last alkali metal and most expensive element.
Alkaline earth metals C. Francium is the most reactive metal on the periodic table. All alkali metals have one valence electron.
Helium is the least reactive metal. This makes Francium the most reactive followed by cesium rubidium potassium sodium and lithium. However francium is a man-made element and only minute quantities have been produced so for all practical purposes the most reactive metal is cesium.
Caesium is the most reactive metal in the periodic table so much that working with this metal often ends in explosions. The most reactive metal on the periodic table is francium. Updated October 08 2019.
The least reactive elements are the noble gases. The least reactive elements are the noble gases. The most reactive metal is francium the last alkali metal and most expensive element.
2 Na s 2 H 2 O l 2 NaOH aq H 2 g Metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. It is predicted francium would react even more vigorously. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide.
Experimentally speaking cesium caesium is the most reactive metal. Reactivity can be defined as the measure of how readily a chemical species will participate in a reaction and form chemical bonds. This makes it easier to remove the electron and makes the atom more reactive.
The most reactive metal in the periodic table is francium. See full answer below. Francium belongs to the alkali metals a group on the periodic table whose members are all highly reactive.
The metal is also radioactive and one of the rarest naturally occurring elements. Become a member and unlock all Study Answers Try it risk-free for 30. In fact naturally occurring francium is so rare that the metal must be synthetically created in a laboratory.
Metalloids in Physics if youre in doubt about the correctness of the answers or theres no answer then try to use the smart search and find answers to the similar questions. Cesium reacts explosively with water. Therefore for practical purposes Cesium is often regarded as the most reactive metal.
Fluorine is a halogen which is Group 17 on the periodic table and the halogens are the most reactive. Francium is almost non-existent in nature so cesium is the most reactive metal of those observed. Find an answer to your question The most reactive metals are theA.
Francium however is a laboratory-produced element and only minute quantities have been made so for all practical purposes the most reactive metal is cesium. The alkali metals become more reactive as you move down in the groups order in the periodic table. The most reactive element is fluorine the first element in the halogen group.
EXAMPLE OF A PHYSICAL CHANGE. BENDING A PAPER CLIP.
Physical Changes Chemical And Physical Changes Of Matter
Physical Change Examples Examples of physical changes include.
/TC_608336-examples-of-physical-changes-5aa986371f4e1300371ebebb.png)
Which is an example of a physical change. EXAMPLE OF A PHYSICAL CHANGE. Examples of physical changes are boiling melting freezing and shredding. A physical change involves a change in physical properties.
Dissolving salt in water is an example of a physical change. EXAMPLE OF A PHYSICAL CHANGE. Dissolving sugar and water.
Melting an ice cube. Examples of Physical Changes. Remember the appearance of matter changes in a physical change but its chemical identity remains the same.
The explosion of fireworks c. The shaping of a gold bar b. Physical change involved tearing breaking grinding a state of matter changing the state of matter and dissolving a substance.
C Lead is melted into a liquid to form pellets. Examples of physical properties include melting transition to a gas change of strength change of durability changes to crystal form textural change shape size color volume and density. Shaking up a bottle of Italian dressing.
Which of these is an example of a chemical change. Some examples of physical change are freezing of water melting of wax boiling of water etc. This is an example of.
Mixing sand and water. CRUSHING AN ALUMINUM CAN. MIXING OIL AND VINERGAR.
Generally physical changes do not involve the production of energy. A simple example of a physical change is an ice cube melting. A physical change is said to occur when something changes form but keeps the same chemical composition.
An example of a physical change is the process of tempering steel to form a knife blade. Most of the time a physical change is the result of the spatial rearrangement of atoms that make up the object. Crushing an empty milk carton.
One example of a physical change is a. When an ice cube melts its constituent molecules change their arrangement and gain some properties like flow or lose some properties like definite shape. The initial substance still exists but only in a different form.
Examples of chemical changes are burning cooking rusting and rotting. A chemical change results from a chemical reaction while a physical change is when matter changes forms but not chemical identity. A few examples of chemical change are digestion of food burning of coal rusting etc.
Some examples of physical change include ice melting alcohol evaporating iron rusting and salt water. Which of these is an example of a physical change. Here are some examples of physical changes you might see.
THIS SET IS OFTEN IN FOLDERS WITH. Physical changes are all around us happening constantly and are easily noticed. Your kitchen is one of the most common places you probably see physical changes happen on a regular basis.
Which of these is an example of a physical change. A Wood is burned in a campfire and is turned into ash and smoke. Which scenario is an example of a physical change.
List some examples of physical change. Freezing water to make ice. Physical changes involve an object or substance changing shape or state of matter.
Boiling pasta to make it soft. D Yeast turns sugar into energy water and carbon dioxide gas. Often physical changes can be undone if energy is input.
Hydrochloric acid is added to a beaker containing a piece of zinc. B A piece of metal is left in the rain and forms rust. Examples of Physical Changes.
Crumpling a sheet or paper a good example of a reversible physical change Breaking a pane of glass the chemical composition of the glass remains the same. Melting ice to make water. The melting of in Chemistry if there is no answer or all answers are wrong use a search bar and try to find the answer among similar questions.
Zinc chloride is formed and hydrogen gas is released. Get an answer to your question Which of the following is NOT an example of a physical changea. Dissolving salt in water.
Even though it now has a different physical property its still the same object or substance its molecules are still the same. It is easy to differentiate between a physical and chemical change. Freezing or solidification refers to the withdrawal of heat from a substance and changes it from a liquid to a solid-state Smoke is a hot vapor that is made up of liquid particles gases and carbonaceous matter.
Common Examples of Physical Changes in Your Kitchen. The salt crystals have changed their physical not chemical state and if you evaporate the water by boiling it the salt will recrystallize and magically appear in the bottom of the pot. Dissolving sugar in your coffee.
Physical changes are all around us.
An example of a chemical change is the reaction between sodium and water to produce sodium hydroxide and hydrogen. Numbers of molecules are conserved in chemical change II.
 Oneclass Which Of The Following Is A Chemical Change A Dissolving Salt In Water B Boiling Water C
Oneclass Which Of The Following Is A Chemical Change A Dissolving Salt In Water B Boiling Water C
Identify the following process.

Which of the following is a chemical change. All of the following are physical properties. Hence they are chemical. Which of the following is a chemical change.
In comparison a chemical change refers to a change in the substance itself. B Digestion of Food ----- Chemical Change. B Wax is melted out of a mold in a kiln.
B Conversion of manure from leaves is a physical change. Boiling point melting point odor. Which of the following changes to a metal is a chemical change.
A B and C are all physical changes because only the physical state of these elements is changed. Cooking food leads to a chemical change in the food. When coal is burned it is combusted to form carbon dioxide water.
What type of changes or processes cause the chemical composition of substances to change into new substances. Which of the following are chemical changes. E A diamond is set into a gold ring.
Chemical Change A chemical change occurs when one substance is transformed into one or more new products via a chemical reaction. I Decaying of wood ii Burning of wood iii Sawing of wood iv Hammering of a nail into a piece of wood. Mixing two cheeses in a bowl.
Which of the following is an example of a chemical change. Burning and decay result in formation of new substances and they cannot be reversed. Making a snowman The formation of a gas resulting from the escape of high-energy particles from the surface of a liquid is known as Select one a.
Broiling a streak on a grill is a method of cooking a steak. This is an example of a chemical change because the end products are chemically different from the substances before the chemical reaction. Water breaking down into hydrogen and oxygen.
The chemical composition of food particles change when exposed to heat above their. In D the atomic structure of the elements is changed which is why it is a chemical not a physical change. Melting gold O d.
Select all that apply. Examples of physical changes are boiling melting freezing and shredding. Mass is conserved in chemical change.
Which of the following is a chemical change. C Iron pipes coated with zinc do not rust easily. A crumpling a piece of paper into a ball B burning wood in a fireplace C digesting a delicious sandwich D crushing ice for soda.
B burning wood in a fireplace C digesting a delicious sandwich. Can be observed without changing the composition of matter. Bending a steel rod O e.
Fermenting of cheese b. Click hereto get an answer to your question Balanced chemical equations imply which of the following. A i and ii B ii and iii C iii and iv D i and iv Medium.
Hydrochloric acid reacts with potassium hydroxide to produce a salt water and heat. Remember that chemical changes form new substances physical changes do not. Examples of chemical changes are burning cooking rusting and rotting.
This means that a new substance is formed. Because here the chemical composition of the substance changes in A B only. C Plaster of paris is ground to a powder for making a mold.
A Gold metal is formed from gold chloride in solution. D A gold ring is resized to fit a new owner. Which of the following is considered a chemical change.
Most chemical changes are not reversible except via another chemical reaction. Determine if the following changes are chemical or physical. Rusting bmelting cbending dpolishing.
Burning sugar O bcutting a rope O c. Salt dissolves in water. Hence the formation of a new chemicala precipitate choice ais a.
A Cutting of wood into pieces is a chemical change. Numbers of atoms are conserved in chemical change. When light is reflected in water.
So much energy is released that the hydrogen gas released spontaneously burns in the air. Identify the chemical change in the following list. D Water is heated up ---- Physical Change.
Melting of cheese c. A chemical change results from a chemical reaction while a physical change is when matter changes forms but not chemical identity. Often physical changes can be undone if energy is input.
C Freezing of water ----- Physical Change. In a chemical change the number and type of atoms remain constant but their arrangement is altered. PHYSICAL AND CHEMICAL CHANGES DRAFT.
Since we know that If the chemical composition is changing then it is a chemical change and if the chemical composition is remaining. The option which is a chemical change is D. Terms in this set 40 physical.
Burning coal involves a chemical change since the chemical composition of the substance changes. The answer is D Burning Coal.
Electronegativity is the tendency for an atom to attract electronsThere are general trends on the periodic table which you can use to determine the relative electronegativity for different elements but otherwise you can also look at their valence shell and effective nuclear charge to determine their relative electronegativity. Thus Si and P will have lower electronegativity values than C and N.
 The Parts Of The Periodic Table
The Parts Of The Periodic Table
And the element which has the lowest electronegativity value is Francium in 07 ch.
/PeriodicTableElectronegativity-56a12a045f9b58b7d0bca77c.jpg)
Which element has the lowest electronegativity. The Pauling scale is the most commonly used. This value uses the Pauling scale to measure electronegativity. The electronegativity chart describes how atoms can attract a pair of electrons to itself by looking at the periodic table you can identify and determine electronegativity values of elements from 0 to 4.
The chemical elements of the periodic chart sorted by. The element that has the lowest electronegative value is LITHIUM. Electronegativity is the ability of an atom to attract the bonding electrons in a covalent bond.
Francium has an electronegativity of 067 on that scale. The most electropositive element is cesium. Values for electronegativity run from 0 to 4.
What does a low electronegativity mean. A key piece of information they contain is the electronegativity value of each of. You can print the list of elements by hitting the print button below.
Silicon Si and Phosphorus P are both found lower in position relative to carbon and nitrogen when going down a column. Arrange the following elements in order of increasing values of electron affinity ie from most negative to least negative. Print List of Elements.
However no refinements have been made for francium as no experiment has been conducted. Electronegativity refers to the tendency of an element to attract a shared pair of electrons to itself. Which element has the lowest electronegativity.
In the periodic table the element with the lowest electronegativity ie. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. This table is a list of electronegativity values of the elements.
These elements have a theoretical electronegativity of zero. The element which has the highest electronegativity value is Fluorine with 398 ch. The alkali metals as a group have the lowest electronegativities with the values falling as the atomic number increases so the winner is caesium cesium with a Pauling score of 079.
19 - Atomic Mass. Check Answer and Solution for above question from C. Electronegativities of the elements data page The electronegativity of francium was chosen by Pauling as 07 close to that of caesium also assessed 07 at that point.
90 - Melting point. Non metals generally have high electronegative values while metals have low electronegative values. 89 - Atomic number.
Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The Periodic Table contains a lot more information than merely the names of each of the chemical elements. Of the group 13 elements the element with lowest electronegativity is A B B Tl C Al D In.
The table gives values on the Pauling scale which have no units. 11 - Boiling point. Which element hast the highest electronegativity.
It can also be used to predict if the resulting molecule will be polar or nonpolar. Which element has the lowest first ionization energy. Electronegativity in the periodic table.
Which element consists of positive ions immersed in a sea of mobile electrons sulfur nitrogen calcium chlorine. Fluorine 398 is the most electronegative element. The lowest electronegativity of the element from the following atomic number is 358 100 LIKES.
The element with the lowest electronegativity value is francium which has an electronegativity of 07. The Allen scale assigns the lowest electronegativity to cesium with a value of 0659. Atomic number - Name alphabetically.
Thus fluorine is the most electronegative element while francium is one of the least electronegative. Which element has the lowest electronegativity Oxygen Flourine Carbon Nitrogen. The base value of hydrogen was later increased by 010 and caesiums electronegativity was later refined to 079.
None of these elements is an exception to the general trends of electron affinities. Which element has the lowest electronegativity. In fact cesium belongs to the alkali group of metals which are the most.
The elements that have the lowest electronegativity are the VIII A elements or noble gases. In the valence shells of lithium carbon bromine and fluorine. What types of bonds are formed when two non-metals combine.
These elements are stable in their electron configuration there is not force moving the noble gases to gain any electrons.
An example of an endothermic and exergonic reaction is C 6 H 12 O 6 6 H 2 O 12 H 2 6 CO 2 r H 627 kJmol r G. The enthalpy increase H 0 in a hypothetical strongly endothermic reaction usually results in G H-TS 0 which means that the reaction will not occur unless driven by electrical or photon energy.
 Example Of Endothermic Reaction With Chemical Equation Brainly In
Example Of Endothermic Reaction With Chemical Equation Brainly In
A few examples of the endothermic process are photosynthesis evaporating liquids melting ice dry ice alkanes cracking thermal decomposition ammonium chloride in water and much more.
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
Which is an example of an endothermic reaction. Freezing the phase transition from liquid to solid form is an exothermic process because energy in the form of heat is emitted in the process. In this process plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. Mechanism of endothermic reactions.
Neutralization is a chemical reaction also called a water forming reaction in which an acid and a base or alkali soluble base react and produce a salt and water solution H 2 OIn other words it can be said that neutralization is the combination of hydrogen ions H and hydroxide ions O H or oxide ions O 2 to form water molecule H 2 OIn the process a salt is formed. Once ice has become liquid it will evaporate as vapor which will still be an endothermic process Cooking an egg - this is also a process that involves absorption of heat energy. A reaction that takes in heat from the surroundings.
An endothermic reaction happens when more energy is required to break or dissolve the bonds in the reactants than what is released when a product is formed by its new bond. Melting of ice is also an example of an endothermic reaction. Photosynthesis is an endothermic reaction.
Endothermic processes Melting ice - when ice melts it takes in energy from the outside region causing it to become liquid. The heat energy received by ice from its surroundings makes it melt into water. A chemical reaction that involves a gain of heat energy from the surroundings resulting in a drop in the temperature of the surroundings is called an endothermic reaction.
The temperature of the surroundings decreases. The salt dissociates into ammonium NH 4 and chloride Cl ions. A chemical reaction that works only if heat is absorbed is an example of a reaction that would be described as endothermic.
In these cases the contact surface is very much increased. A cold pack used in sports gets cold when the chemical inside mixes with the water bag and new bonds form causing a decrease in temperature. Photosynthesis Another true well-known endothermic reaction is photosynthesis.
The thermal decomposition of limestone. Energy in the form of sunlight is absorbed during the reaction. Fusion of snow on a warm windshield especially for heavy snow-wet.
When ammonium chloride NH 4 Cl is dissolved in water an endothermic reaction takes place. Examples Endothermic Reaction Examples. Endothermic Chemical Reactions.
An example of an endothermic process with which everyone knows is sweating the process by which the body produces water on the skin as a cooling strategy. Examples Fusion of ice. Examples of Endothermic Reactions Dissolution of Ammonium Chloride in Water The dissolution of ammonium chloride in water results in absorption of heat energy from the surrounding environment hence a drop in temperature of the solution.
In our daily life it is also possible to find examples of such reactions. Endothermic Reactions The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. In chemistry laboratories it is common to deal with endothermic reactions such as hydrolysis of water or the production of molecular chlorine from hydrochloric acid.
Photosynthesis is an example of an endothermic chemical reaction. Why is freezing exothermic. In this process plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen.
To simplify the endothermic reaction takes a certain portion of energy in order to work. Quicklime can be used to make steel from iron and also to neutralise soils that are too acid. The reaction equationNH4NO3 s water NH4 aq NO3 aqThis is an example of endothermic reaction because the temperature drops because heat energy is taken in by the reaction mixture.
A good example of an endothermic reaction includes dissolving a salt. It works because water absorbs energy when it changes state from a liquid to a gas. The endothermic reaction makes the water colder and the bag feel frosty and oh-so-good on your swollen ankle.
In industry the breakdown of limestone into quicklime and carbon dioxide is very important. It doesnt have to be table salt nor does the solvent need to be water. Since this physical change cannot occur without the heat input from surroundings this phenomenon is classified as an endothermic process.