The specific enthalpy of fusion more commonly known as latent heat of water is 33355 kJkg at 0 C. Freezing point is the temperature at which any liquid will change its state to a solid.
:max_bytes(150000):strip_icc()/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif) What Is The Freezing Point Of Water
What Is The Freezing Point Of Water
This means that at 0 C water changes from a solid to a liquid.
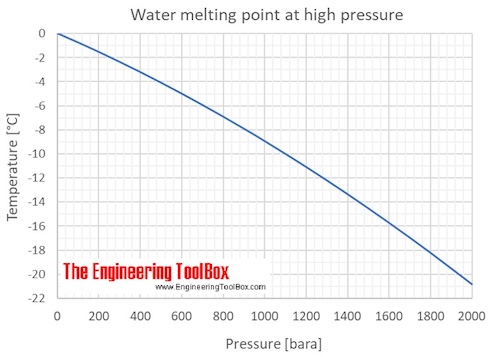
What is the melting point of water. The use of ordinary salt sodium chloride NaCl on icy roads in the winter helps to melt the ice from the roads by lowering the melting point of the ice. Chocolate melts at about 35C. You can demonstrate this effect by cooling very pure water in a freezer in a smooth container to as low as 42 degrees Celcius.
Of common substances only that of ammonia is higher. Will my school be closed for the big snow storm Monday. Melting Point Determination The melting point is determined in a capillary tube.
But when it mixes with other substances then it has a lower melting or freezing point. The melting point of water is dependent of the pressure above the ice solid water and the melting point or freezing temperature decreases with increasing pressure. Under normal conditions when the pressure of the atmosphere is approximately 760 mmHg water boils at 100 o C.
The melting point of water is over 100 k higher than expected by extrapolation of the melting points of other group 6a hydrides here above right shown compared with group 4a hydrides. For h2o melting point decreases with increasing pressure for co2 melting point increases with increasing pressure. This happens at 100 degrees centigrade for water.
However for practical purposes the melting point of pure water ice at 1 atmosphere of pressure is very nearly 0 C which is 32 F or 27315 K. The same amount of energy is required to melt ice as to warm ice from 160 C up to its melting point or to heat the same amount of water by about 80 C. As a result of the interconnected lattice structure of water molecules in its solid form due to hydrogen bonding between molecules a high input of energy is required to transform ice into its liquid form.
The temperature at which a solid turns into a liquid. The melting point is lowered by 185 degrees Celsius if 292 grams of salt are dissolved in each Kg of water called a 05 molal solution of salt. The terms melting point or freezing point are often interchanged depending on whether a substance is being heated or cooled.
When considered as the temperature of the reverse change. Lower the Room temp. This is supercooling and it occurs with many substances including waterUnless there is a nucleus for crystallization you can cool water well below its melting point and it wont turn to ice freeze.
Tungsten has the highest melting point which is 3410 C. Water is a pure substance. It has the same melting and freezing points.
The melting point for water is 0 degrees C 32 degrees F. The boiling point for any material is the temperature point at which the material transforms into the gas phase in the liquid phase. The normal melting point of a substance is defined as the melting temperature at a pressure of one atmosphere equivalent to 101325 bars.
For instance the melting point of ice is 0C or 273K so at this temperature ice will start breaking down as a liquid. Melting Pointedit Water in its ice form has a usually high melting point temperature a value close to 0C. The melting point of a solid and the freezing point of the liquid are normally the same.
Each substance carries its own boiling point. For liquids it is known as the freezing point and for solids it is called the melting point. The melting point of water is 0 C.
The melting point or rarely liquefaction point of a substance is the temperature at which it changes state from solid to liquidAt the melting point the solid and liquid phase exist in equilibriumThe melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. You should write independent equilibrium. Phase diagram for water.
Water has a melting point of 0 C. For instance at room temperature Boiling point of water is 100C at this temperature water molecules start evaporating as vapors. The normal boiling point of water is 100 o C because this is the temperature at which the vapor pressure of water is 760 mmHg or 1 atm.
When the opposite happens and a liquid turns into a solid it is called freezing. The solid and liquid phase of water are in equilibrium at this temperature. The melting point depends slightly on pressure so there is not a single temperature that can be considered to be the melting point of water.
Critical temperatures the maximum temperature at. What is Freezing Point. The Celsius scale was in fact created on the basis of the icewater melting point and the liquid watervapor boiling point.
Boiling Point is the temperature when liquid starts getting transform into vapors form. It differs little from the triple-point temperature because of the steepness of melting lines TM in Figure 1. Melting point is the temperature at which something becomes a liquid.
The melting point of water is the temperature at which it changes from solid ice into liquid water. The Na and Cl dissociate right away when dissolved and so for a 05 molal solution of salt there is a 10 molal concentration of ions. The melting point of any substance depends largely on the standard atmospheric pressure while pressure only has a little effect on a substances freezing point.
By definition 0 C is at the melting point of water at 1 atmosphere pressure. Also the melting point.
The melting point of water is over 100 k higher than expected by extrapolation of the melting points of other group 6a hydrides here above right shown compared with group 4a hydrides. The melting point of any given substance is the temperature at which the substance experiences melting or the moment that the substance changes from a solid into a liquidSome materials like metals glass and other substances which are solid at room temperature have a high melting point.
 Melting Point Of Water 1 And Water 2 As A Function Of The System Download Scientific Diagram
Melting Point Of Water 1 And Water 2 As A Function Of The System Download Scientific Diagram
Water is a liquid thus already melted.

Melting point of water. Celsius or centigrade is a unit of measurement of temperature. The temperature at which a solid turns into a liquid. By definition 0 C is at the melting point of water at 1 atmosphere pressure.
Under normal conditions when the pressure of the atmosphere is approximately 760 mmHg water boils at 100 o C. Select up to 4 products. The melting point of water is the temperature at which it changes from solid ice into liquid water.
Boiling and Melting Point of Water Other contents. The same amount of energy is required to melt ice as to warm ice from 160 C up to its melting point or to heat the same amount of water by about 80 C. The boiling point of water is about on one hundred degrees celsius 100 C at a pressure of 1 atmosphere.
For instance at room temperature Boiling point of water is 100C at this temperature water molecules start evaporating as vapors. For instance the melting point of ice is 0C or 273K so at this temperature ice will start breaking down as a liquid. For h2o melting point decreases with increasing pressure for co2 melting point increases with increasing pressure.
Beside this what is melting and melting point. Phase diagram for water. Boiling and Melting Point of Water Boiling and Melting Point of Water ID.
States of Matter Add to my workbooks 8 Add to Google Classroom. Ordinarily the freezing point of water and melting point is 0 C or 32 F. Please select more than one item to compare.
Melting point temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The freezingmelting point of water is about zero degrees celsius 0 C at a pressure of 1 atmosphere. When considered as the temperature of the reverse change.
Your question should either be about raising the melting point of ice or raising the boiling point of water. 64 matches found for Melting point of water. For liquids it is known as the freezing point and for solids it is called the melting point.
The temperature may be lower if supercooling occurs or if there are impurities present in the water which could cause freezing point depression to occur. Melting point of water Compare Products. Water has a melting point of 0 C.
The melting point of water is dependent of the pressure above the ice solid water and the melting point or freezing temperature decreases with increasing pressure. The boiling point of water is 100 C ie. As heat is applied to a solid its temperature will increase until the melting point is reached.
This means that at 0 C water changes from a solid to a liquid. 1 Product Result. The melting point for water is 0 degrees C 32 degrees F.
Boiling Point is the temperature when liquid starts getting transform into vapors form. The terms melting point or freezing point are often interchanged depending on whether a substance is being heated or cooled. When the opposite happens and a liquid turns into a solid it is called freezing.
However for practical purposes the melting point of pure water ice at 1 atmosphere of pressure is very nearly 0 C which is 32 F or 27315 K. The vapour pressure of water becomes equal to atmospheric pressure at 100 C at sea level. The specific enthalpy of fusion more commonly known as latent heat of water is 33355 kJkg at 0 C.
The melting point depends slightly on pressure so there is not a single temperature that can be considered to be the melting point of water. Of common substances only that of ammonia is higher. The solid and liquid phase of water are in equilibrium at this temperature.
The melting point or rarely liquefaction point of a substance is the temperature at which it changes state from solid to liquidAt the melting point the solid and liquid phase exist in equilibriumThe melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. Melting point is the temperature at which something becomes a liquid. Under certain conditions water may remain a liquid as cold as -40 to -42F.
More heat then will convert the solid into a liquid with no temperature change. To raise the melting point of ice meaning trying to keep ice as a solid at a temperature as high as possible reduce impurities. The melting point of a solid and the freezing point of the liquid are normally the same.
Advanced Search Structure Search. The normal boiling point of water is 100 o C because this is the temperature at which the vapor pressure of water is 760 mmHg or 1 atm.