The pH of an aqueous of the corresponding salt B A will be. Weak electrolytes do not.
 Acids And Bases Ph And Titrations Ppt Video Online Download
Acids And Bases Ph And Titrations Ppt Video Online Download
BaOH2 barium hydroxide.
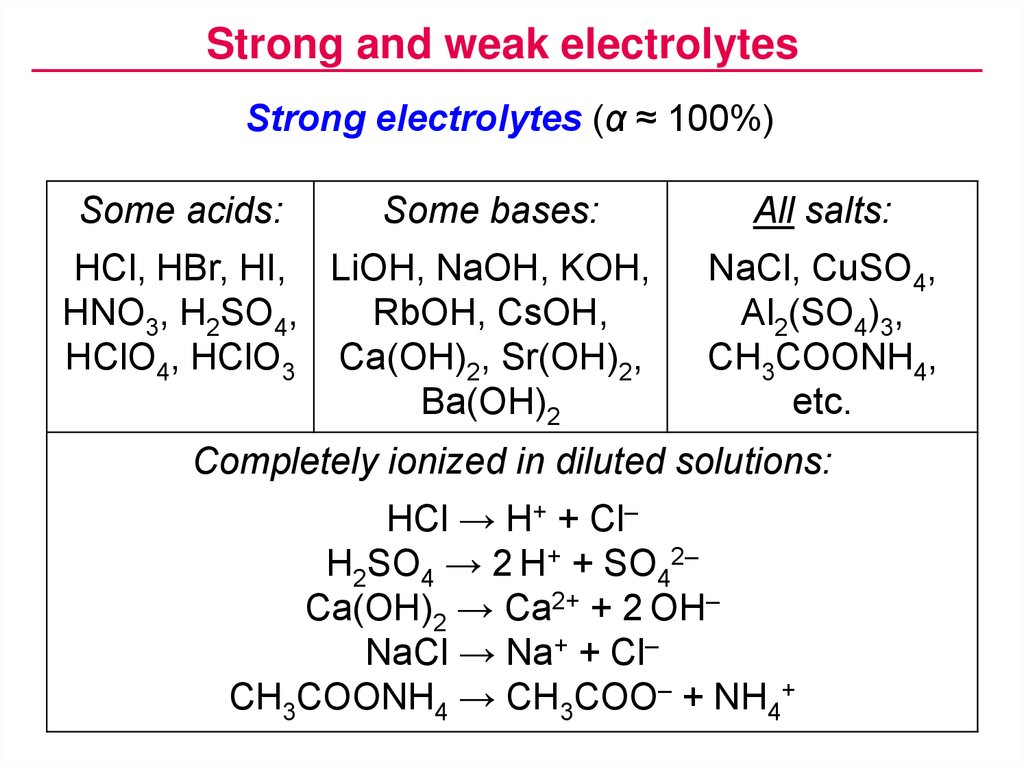
H2so4 strong or weak. H2SO4 sulfuric acid. Strong electrolyte soluble and strong acid Chloric acid. In your first list H2SO4 is usually defined as strong but the ionization is two part.
When the strong acid has been completely. Identify each acid or base as strong or weak. Instructions For Electrolytic Behavior Table Light.
HSO4- already has a negative charge so its much harder to pull of an H. Account for the difference in strength of these two related species. The acids corrosiveness towards other.
MgOH 2 C 5 H 5 N. In this curve there are two break points. Indicate If The Substance Is A Strong Electrolyte Weak Electrolyte Or Non-electrolyte Substances Present In Solution.
Strong dehydrating acids H2SO4 H3PO4 favor elimination dehydration in alcohols. Because they are strong acids they readily protonate the alcohol thereby converting a poor leaving group OH- into a good leaving group HOH however the anions produced after protonation of the alcohol HSO4- or H2PO4- are very poor nucleophiles and can. H2SO4 H HSO4-.
Strong electrolyte ionic compound CaBr2. Hydrofluoric acid while a weak acid would pass through your hand and attack your bones. Sulfuric acid American IUPAC spelling or sulphuric acid traditional British spelling also known as oil of vitriol is a mineral acid composed of the elements sulfur oxygen and hydrogen with molecular formula H 2 SO 4It is a colourless and viscous liquid that is soluble in water and is synthesized in reactions that are highly exothermic.
HSO4- H SO4 but even without extra water it goes 95. Indicate If The Bulb Brightness Is Bright Dim Or No Light Observed Electrolyte. Therefore we refer to H2SO4 as a strong acid BUT only for the first proton that is released.
The HSO4- ion is a weak acid and only partially dissociates. For H2SO4 the proton leaves a neutral molecule. For that reason a 1 M solution of H2SO4 will NOT produce a 2M hydrogen ion concentration but instead it will produce just a little over 1 M hydrogen ions.
Conductometric titration of a weak acid acetic acid vs. - Hydrochloric Acid HCl - Hydrobromic Acid HBr - Sulfuric Acid H2SO4 - Perchloric Acid HClO4 - Nitric Acid HNO3 - Hydroiodic Acid HI - Periodic Acid HIO4 - Chloric Acid HClO4 Strong Bases. The second ionization may not go 100 unless extra water is used.
Because MgOH 2 is listed in Table 122 Strong Acids and Bases it is a strong base. HNO2 nitrous acid. A Strong Base or a Weak Base.
The p K b of a weak base B O H is 4. Strong Weak And Nonelectrolytes. ACID BASE HCI CI- H2SO4 HSO4- - Negligible Strong- HNO3 NO3- H30 H20 HSO4 so2- H2SO3 HSO3- H3PO4 H2PO4 HF F- CH3COOH CH3CO0- H2CO3 HCO- Weak H2S HS- Weak- HSO3- SO32- H2PO4- HPO42- HCN CN- NH4 NH3 HCO3- Co22- HPO42- PO43- H20 On- HS- S2- Strong Negligible - On- 02- ACID STRENGTH BA SE STRENGTH.
H2SO4 is a strong acid but HSO4- is a weak acid. CH3COOH acetic acid. Strong electrolyte strong acid H2SO4.
Strong electrolyte ionic compound AlCl3. Tell What Substance Is Dissolved. HI hydroiodic acid.
Answer to H2SO4 is a strong acid but is a weak acid. Mixture of a Strong Acid and a Weak Acid vs. HF hydrofluoric acid.
Strong electrolyte ionic compound Perchloric acid. Account for the difference in strength of these two related species. Because HCl is listed in Table 122 Strong Acids and Bases it is a strong acid.
HCN hydrocyanic acid. - Sodium Hydroxide NaOH. While acids tend to be corrosive the strongest superacids carboranes are actually not corrosive and could be held in your hand.
HCOOH formic acid. The first break point corresponds to the neutralization of strong acid. HBr hydrobromic acid.
The nitrogen in C 5 H 5 N would act as a proton acceptor and therefore can be considered a base but because it does not. The dissociation constant of two acids H A 1. Strong electrolyte strong acid Potassium hydroxide.
A strong acid or base is one that is completely ionized in a solution. Strong electrolytes ionize completely upon solvation. H2SO4 is a strong acid.
1 H2SO4 -- H HSO4-The remaining bisulfate ion HSO4- is a weak acid and only partially dissociates. A weak base NH 4OH 5. Choose strong acids from the following CH3COOH H2SO4HNO3H2CO3 - 5429451.
The reason is that sulfuric acid is highly corrosive while acetic acid is not as active. Strong electrolyte soluble and strong acid HNO3. Follow up.
It is a strong acid only for the first hydrogen ion that is produced.