The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. The ChemTeam will try to use several different weak acids in the examples to follow.
/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png) List Of Common Strong And Weak Acids
List Of Common Strong And Weak Acids
Nitric acid HNO 3.
List of weak acids and bases. As a result they are commonly found in various household applications especially as cleaners and in the kitchen. CaOH 2 - calcium hydroxide. H 2 CO 3.
Furthermore weak acids and bases are very common and we encounter them often both in the academic problems and in everyday life. The time honored example weak acid is acetic acid. Acetic acid also known as ethanoic acid is a weak acid with the chemical formula CH 3 COOH.
Acetic acid is a weak acid because it only partially dissociates into its constituent ions when dissolved in water. There are very few strong bases Table PageIndex1. The strong acids are hydrochloric acid nitric acid sulfuric acid hydrobromic acid hydroiodic acid perchloric acid and chloric acid.
TextHF is a weak acid. The nitrogen in C 5 H 5 N would act as a proton acceptor and therefore can be considered a base but because it does not. Common Strong Bases Weak Bases Strong Acids and Polyprotic Acids.
Various common acids and bases that you should memorize. Weak acids and bases are only partially ionized in their solutions whereas strong acids and bases are completely ionized when dissolved in water. If it is less than 100 ionized in solution it is a weak base.
Weak bases do not furnish OH - ions by dissociation. N ext comes acetic acid a primary constituent of vinegar. RbOH - rubidium hydroxide.
It is known to be the active component of vinegar which is a 4 7 solution of acetic acid in water. CH 3 COOH H 2 CO 3. Strong acids are listed at the top left hand corner of the table and have Ka values 1 2.
Complete List of Acids Complete List of Bases Molar to Mass Concentration Converter Molar Mass Calculator Cations Anions List Dilution Calculator Molarity Calculator Compound Prefixes Water Insoluble Compounds Compound Quiz Concentration Solution Unit Converter English Word Search. When you combine acids and bases into a mixture they neutralize each other. HCl HNO 3 H 2 SO 4.
FEEL FREE TO EDIT OR ADD MORE TO THE SET. Strong and Weak Bases. An acid is a compound that dissolves in water to release hydrogen ions.
NH 3 CH 3 CH 2 NH 2. CsOH - cesium hydroxide. Bases are also molecules that are bitter in taste and have opaque coloring.
Weak acids and bases. The issue is similar with bases. The most common example among weak bases is ammonia NH 3.
BaOH 2 - barium hydroxide. - poor conductors - low value for current passing. Acids are molecules that release hydrogen ions or protons in a solution.
- good conductors Weak bases. H 3 PO 4. Acids and bases are generally chemically active in that they can react with many other substances.
Terms in this set 25 HCl hydrochloric acid strong acid. Examples of weak bases include ammonia NH 3 and diethylamine CH 3 CH 2 2 NH. They are generally sour and can dissolve metals.
Group 1 hydroxides ie NaOH etc or lower group 2 hydroxides BaOH 2. In fact it has its own abbreviation of HAc where H means hydrogen and Ac means acetate. All strong bases are OH compounds.
Weak acid and weak base A weak acid or base is one where only a small percentage of molecules dissociate to form ions in solution. - good conductors - large value for current passing. Because HCl is listed in Table 122 Strong Acids and Bases it is a strong acid.
SrOH 2 - strontium hydroxide. The only weak acid formed by the reaction between hydrogen and a halogen is hydrofluoric acid HF. A weak acid or base is one where only a small percentage of molecules will dissociate to form ions in solution.
Identify each acid or base as strong or weak. Acetic acid being a weak acid when released into the air is broken down naturally by sunlight. The reason for this is that strong acids and bases are 100.
Weak acid - an acid that only partly ionizes in water producing hydrogen ions. MgOH 2 C 5 H 5 N. C5H5N pyridine Remember any base that dissolves in water is an alkali and must have a pH above 7.
Acids and bases are either strong or weak. It has many uses ranging from manufacturing inland dyes pesticides food preservatives rubber plastic and many more. A strong base is a base that is 100 ionized in solution.
Like weak acids weak bases do not completely dissociate in aqueous solution. Weak Acids - Tylenol acetaminophen K a - 12 x 10 -10 and Aspirin acetylsalicylic acid or ASA K a - 327 x 10 -4. Any base not listed is a weak base.
Because MgOH 2 is listed in Table 122 Strong Acids and Bases it is a strong base. Acid with values less than one are considered weak. Also we run into a bit of a technicality in the language.
Strong acids - hydrochloric acid HCl. Some common weak acids and bases are given here. Diethylamine CH 3 CH 2 2 NH.
Most weak bases are anions of weak acids. CH 3 NH 2.
Strong And Weak Acids And Bases Acids Are Pretty Basic
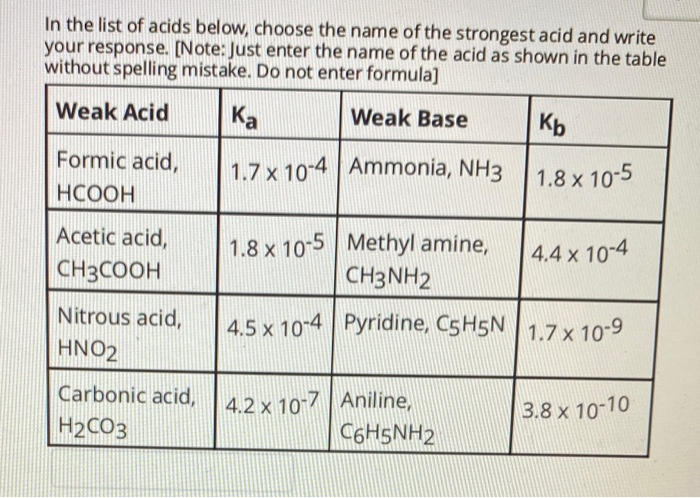 Solved Im Confused With How You D Go About Determining If Chegg Com
Solved Im Confused With How You D Go About Determining If Chegg Com
 Super Trick To Learn Example Of Strong Acid Strong Base Weak Acid Weak Base Type Of Salt Ionic Youtube
Super Trick To Learn Example Of Strong Acid Strong Base Weak Acid Weak Base Type Of Salt Ionic Youtube
 What Are Some Examples Of Weak Bases Quora
What Are Some Examples Of Weak Bases Quora
 List Of Common Strong And Weak Acids
List Of Common Strong And Weak Acids

 List Of Strong Weak Acids Bases Chemistry Basics Chemistry Organic Chemistry
List Of Strong Weak Acids Bases Chemistry Basics Chemistry Organic Chemistry
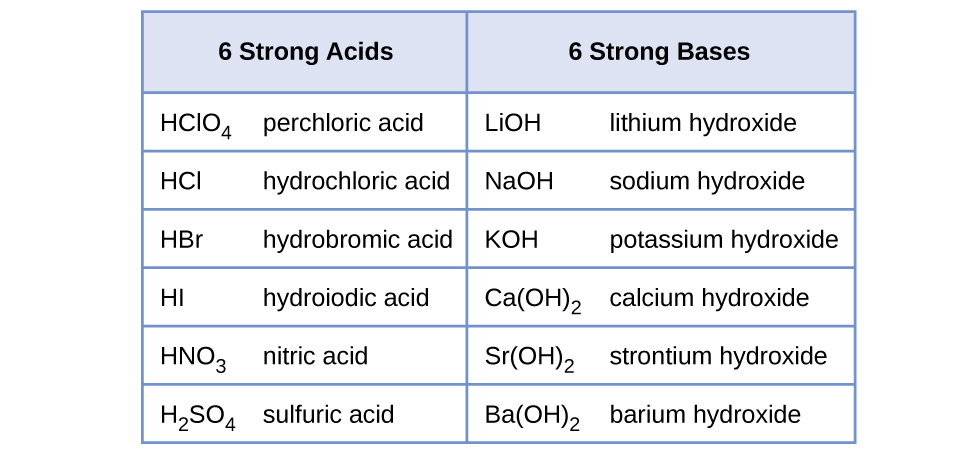 14 3 Relative Strengths Of Acids And Bases Chemistry
14 3 Relative Strengths Of Acids And Bases Chemistry
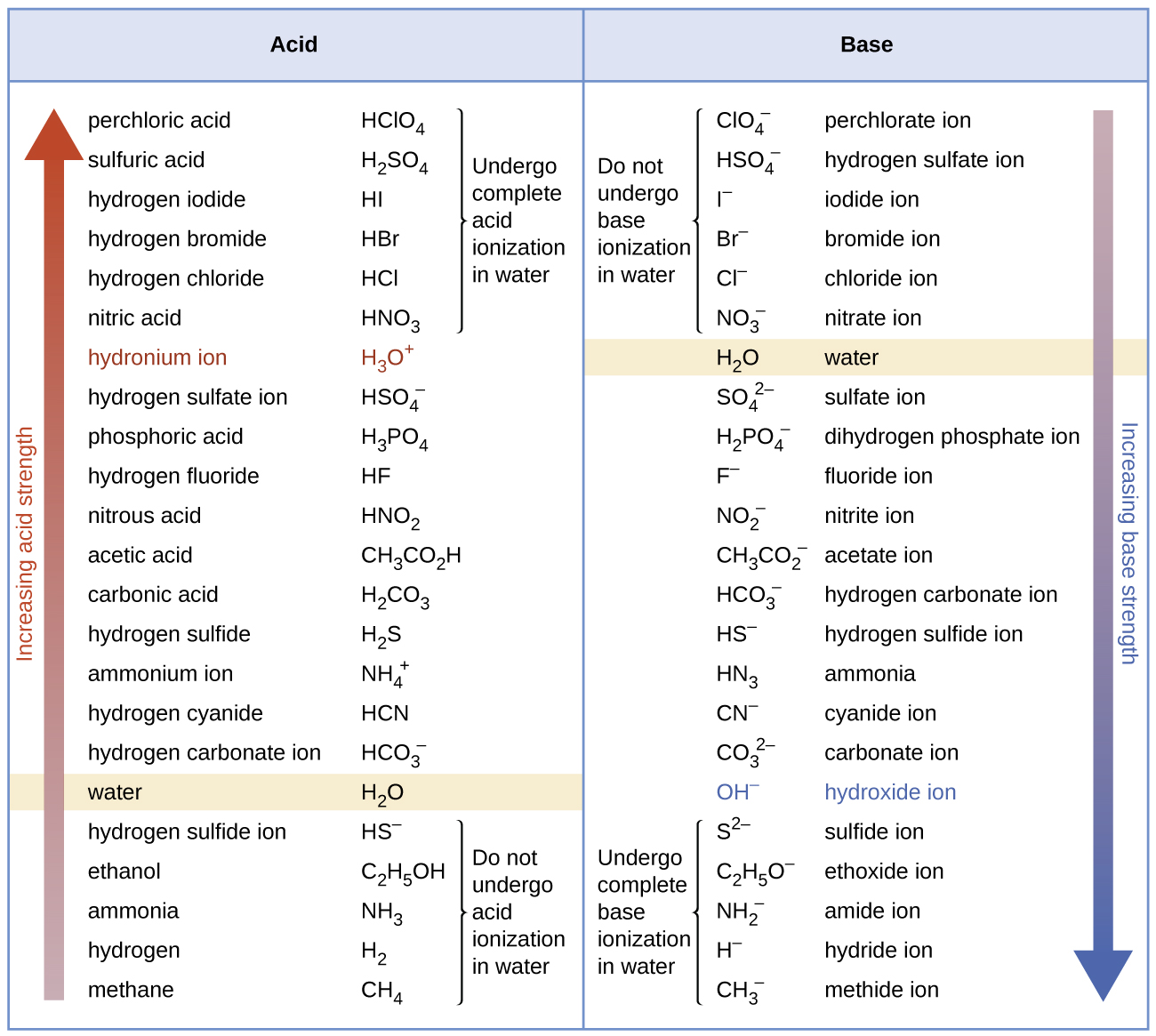 15 4 Equilibria Involving Weak Acids And Bases Chemistry Libretexts
15 4 Equilibria Involving Weak Acids And Bases Chemistry Libretexts
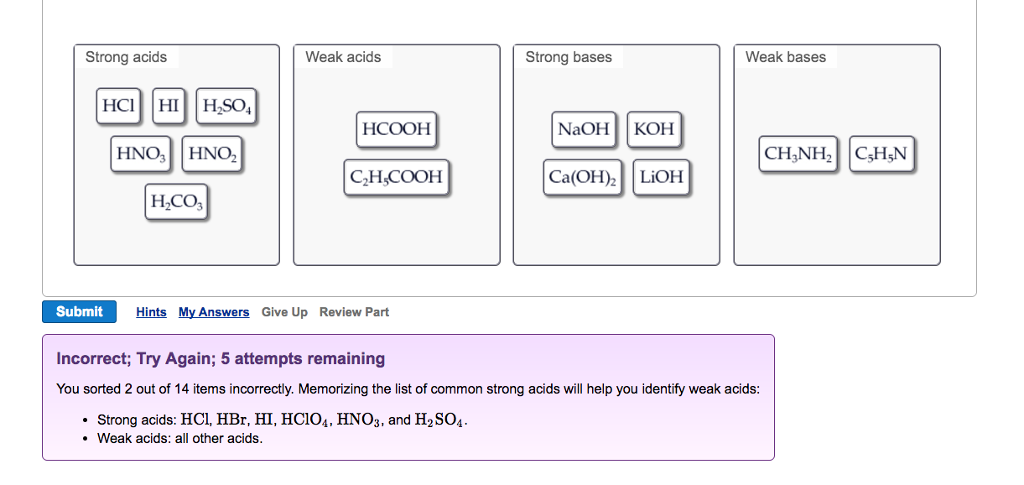 Solved Strong Acids Weak Acids Strong Bases Weak Bases Na Chegg Com
Solved Strong Acids Weak Acids Strong Bases Weak Bases Na Chegg Com
/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png) List Of Common Strong And Weak Acids
List Of Common Strong And Weak Acids
 What Is The Pka Range For Weak Acids And Bases Chemistry Stack Exchange
What Is The Pka Range For Weak Acids And Bases Chemistry Stack Exchange
 Strong Acids And Bases Mcat Chemistry Cheat Sheet Study Guide Studypk Chemistry Education Teaching Chemistry Chemistry Classroom
Strong Acids And Bases Mcat Chemistry Cheat Sheet Study Guide Studypk Chemistry Education Teaching Chemistry Chemistry Classroom
quiz for toddlers
Kids Quiz 100 Easy Children's Quiz Questions with Answers . 1 – What is the Yellow part of an egg called? 2 – What is the first na...

ads
